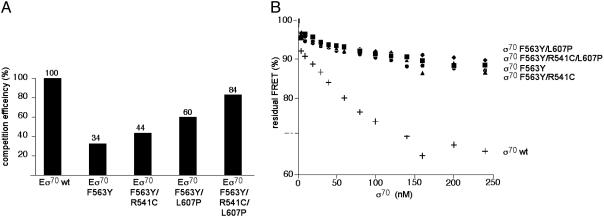
Fig. 4.
Effects of substitutions in σ70 on AsiA binding in the context of RNAP holoenzyme (A) and free σ70 (B). (A) The indicated RNAP holoenzymes were added to a ternary complex containing RNAP core and fluorescently labeled σ70 and AsiA. The decrease in FRET signal was determined and converted to competition efficiency (assumed to be 100% for wild-type RNAP holoenzyme). (B) A complex between fluorescently labeled σ70 and AsiA was formed and the effect of the addition of increasing concentrations of unlabeled wild-type or mutant σ70 subunits on the FRET signal was determined (plus signs, wild-type σ70; circles, σ70 F563Y; triangles, σ70 F563Y/R541C; diamonds, σ70 F563Y/L607P; squares, σ70 F563Y/R541C/L607P). The decrease in FRET signal reflects the ability of each added σ70 to compete for labeled AsiA.