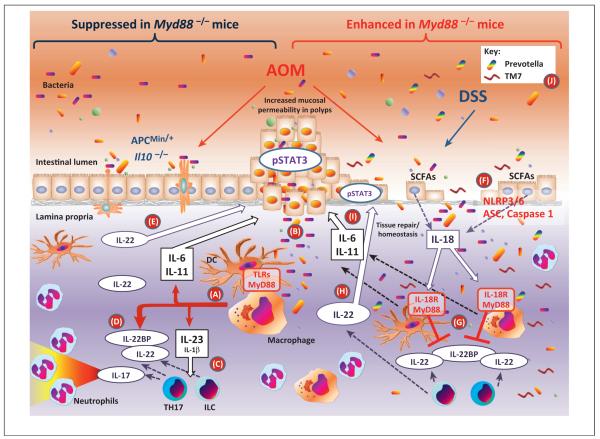
Figure 3.
Divergent roles of myeloid differentiation factor 88 (MyD88) in colon carcinogenesis. MyD88 deficiency in the models of colon carcinogenesis shown on the left side of the figure in which mucosa damage is limited [e.g., repeated treatments with the azoxymethane (AOM) carcinogen, ApcMin/+ or Il10−/− mice] and protects against tumor growth, probably by limiting in hematopoietic cells the inflammatory response downstream of Toll-like receptors (TLRs) triggered by commensal bacteria or their products (pathway A) [24,33,81,82]. Once polyps are formed, they are characterized by increased permeability allowing bacterial trans-mucosal translocation (B) and tumor promotion due to an increased inflammatory response, dominated by the production of interleukin-23 (IL-23) that induces T helper 17 (Th17) cells and innate lymphoid cells (ILCs) to produce IL-22 and IL-17 (C) [93]. IL-22 is important in mucosal homeostasis and repair [92] and is regulated at the level of production as well as by the amount of IL-22 binding protein (IL-22BP) produced (D) [91]. However, persistence of IL-22 after mucosal repair prolongs epithelial cell proliferation favoring polyp formation (E) [91]. Thus, IL-22-deficient mice have decreased and IL-22BP-deficient mice have increased carcinogenesis in these models [91]. In the AOM/dextran sulfate sodium (DSS) model of colon carcinogenesis shown on the right side of the figure, the extensive mucosal damage and erosion induced by DSS allow massive trans-mucosal microbial translocation with an excessive inflammatory response [24]. Microbes, in part through the release of short chain fatty acids (SCFAs) [103], induce epithelial cells to produce IL-18 (F) that has a mucosa protective effect by blocking the production of IL-22BP (G), thus increasing the bioavailability of IL-22 (H) for mucosa tissue repairs [91]. MyD88-deficient mice lack the IL-18 protective effect because of the role of MyD88 in IL-18R signaling while they are able to produce colon damaging and tumor-inducing inflammatory cytokines such as IL-11 and IL-6 (I) even in the absence of TLR and IL-1R signaling [37]. Thus, MyD88-deficient mice have a dramatically increased susceptibility to AOM/DSS-induced colitis and carcinogenesis [37,83–85]. This phenotype is in part reproduced by mice unable to produce IL-18 (Il18−/−), to process pro-IL-18 (Nlrp3−/−, Nlrp6−/−, ASC−/−, or Caspase1−/−) or to signal in response to IL-18 (Il18r−/− ) [89,90]. Also, deficiency of IL-18 functions results in dysbiosis (J) with expansion of colitis-inducing bacteria such as TM7 and Prevotella spp. [90]. In the AOM/DSS model, IL-22-deficient mice show increased and IL-22BP-deficient mice decreased susceptibility to colitis-associated cancer [91].