Abstract
Lung cancer in never smokers, which has been partially attributed to household solid fuel use (i.e coal), is etiologically and clinically different from lung cancer attributed to tobacco smoking. To explore the spectrum of driver mutations among lung cancer tissues from never smokers, specifically in a population where high lung cancer rates have been attributed to indoor air pollution from domestic coal use, multiplexed assays were used to detect >40 point mutations, insertions, and deletions (EGFR, KRAS, BRAF, HER2, NRAS, PIK3CA, MEK1, AKT1, and PTEN) among the lung tumors of confirmed never smoking females from Xuanwei, China [32 adenocarcinomas (ADCs), 7 squamous cell carcinomas (SCCs), 1 adenosquamous carcinoma (ADSC)]. EGFR mutations were detected in 35% of tumors. 46% of these involved EGFR exon 18 G719X, while 14% were exon 21 L858R mutations. KRAS mutations, all of which were G12C_34G>T, were observed in 15% of tumors. EGFR and KRAS mutations were mutually exclusive, and no mutations were observed in the other tested genes. Most point mutations were transversions and were also found in tumors from patients who used coal in their homes. Our high mutation frequencies in EGFR exon 18 and KRAS and low mutation frequency in EGFR exon 21 are strikingly divergent from those in other smoking and never smoking populations from Asia. Given that our subjects live in a region where coal is typically burned indoors, our findings provide new insights into the pathogenesis of lung cancer among never smoking females exposed to indoor air pollution from coal.
Keywords: EGFR, KRAS, lung cancer, never smoking, China, driver mutations, tumor tissue
Introduction
Lung cancer has been the most common cancer in the world for over two decades [1]. Globally, about 53% of lung cancer cases in women and 15% of lung cancer cases in men are not attributable to tobacco use [2]. Evidence suggest that lung cancer in never smokers has unique risk factors, clinical features, and histological distributions when compared to lung cancer cases attributed to smoking tobacco, with lung cancer in never smokers presenting predominately as adenocarcinoma and in females [3, 4].
Given that most women throughout Asia historically do not smoke, they constitute an ideal study population which can enable the elucidation of risk factors for developing never smoking lung cancer. Interestingly, never smoking women in certain regions of Asia experience some of the highest lung cancer rates in the world [5, 6]. These rates have been partially attributed to known environmental risk factors such as fuel combustion byproducts from indoor heating and cooking and environmental tobacco smoke [5, 7]. Xuanwei, China is a unique region in which to study these environmental exposures as it has the highest female prevalence of lung cancer in China and a majority of women are never smokers [6, 8, 9]. Nearly all Xuanwei women have substantial exposure to indoor air pollution from domestic fuel combustion for heating and cooking [10, 11], an established risk factor for lung cancer [12].
Driver mutations occur in genes that encode signaling proteins that play key roles in the regulation of cell death and proliferation [13]. Many driver mutations have been found in lung cancer and are now used to classify tumors at the molecular level. Given the dramatic and prolonged benefit in patients with EGFR mutant tumors treated with EGFR tyrosine kinase inhibitors [14], most reports have focused on EGFR mutations; however, few studies have characterized tumors specifically from never smokers [15, 16], and even fewer studies have evaluated the impact of environmental exposures on these mutation patterns. To date, most reports have evaluated mutations in relation to environmental tobacco smoke and radon exposures [17–19]. For this reason, this study sought to evaluate EGFR, KRAS, BRAF, HER2, NRAS, PIK3CA, MEK1, AKT1, and PTEN driver mutations present in tumor samples collected in Xuanwei to produce new insights into the pathogenesis of lung cancer among never smoking females exposed to indoor air pollution from coal.
Methods
Lung cancer patients presenting to hospitals in Xuanwei, China were eligible for participation in our ongoing study. During surgery, a piece of tumor from the lung was extracted, formalin-fixed, and paraffin embedded. This study was reviewed and approved by the National Institutes of Health's Office of Human Subjects Research.
In total, 40 formalin-fixed paraffin embedded (FFPE) tissues were collected from never smoking female lung cancer cases in Xuanwei. Expert consensus review (by KDJ and JS) of the FFPE tissue samples from this series of 40 never smoking female lung cancer cases was conducted to determine histology and to identify viable tumor areas for dissection and subsequent nucleic acid isolation. The selected tumor areas for use in DNA isolation were required to contain at least 50% viable tumor cells. Of the 40 cases, 32 (80.0%) of these never smoking female cases were adenocarcinomas, seven (17.5%) were squamous cell carcinomas, and one (2.5%) was an adenosquamous carcinoma.
Two multiplexed assays were used to evaluate the FFPE tissue DNA for more than 40 recurrent mutations in 9 genes relevant to existing and emerging targeted lung cancer therapies. First, the amplification of DNA through Applied Biosystem’s SNaPshot was used to detect 38 different recurrent point mutations in 8 driver genes (EGFR, KRAS, BRAF, NRAS, PIK3CA, MEK1, AKT1, and PTEN). This platform involved multiplexed amplification of DNA targets by the polymerase chain reaction (PCR) with unlabeled oligonucleotide primers, multiplexed single-base primer extension with fluorescently-labeled dideoxynucleotides, and analysis of labeled primer-extension products by capillary electrophoresis. The second assay was a separate PCR-based sizing technique that simultaneously assesses tumors for recurrent insertions in EGFR and HER2 and deletions in EGFR that would not be comprehensively detected by the SNaPshot technique. This assay was used for EGFR exon 19 deletions, EGFR exon 20 insertions, and HER2 exon 20 insertions. Compared to direct sequencing, these assays offer higher analytical sensitivity and reduced complexity. They also provide a robust and accessible approach for the rapid identification of important mutations in lung cancer [20].
Chi-squared and Fischer's exact tests were used to compare the number of specific mutations between subgroups. Two-sided p-values are reported.
Results
The 40 FFPE tissues collected among female never smoking lung cancer cases in Xuanwei were predominantly adenocarcinomas (80.0%) and to a lesser exent squamous cell carcinomas (17.5%). The mean (±standard deviation) age of these cases was 46.5 years old (±10.0 years) (Table 1).
Table 1.
Tumor and patient characteristics of never smoking female lung cancers from Xuanwei, China
| Adenocarcinoma | Squamous Cell Carcinoma | Adenosquamous | All Lung Cancer Cases | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n = 32 | n = 7 | n = 1 | n = 40 | ||||||
| n | % | n | % | n | % | n | % | ||
| Age (mean ± std) | 46.6 ± 10.1 | 45.7 ± 10.6 | 48.0 ± not applicable | 46.5 ± 10.0 | |||||
| Differentiation | |||||||||
| Well differentiated | 6 | 18.8 | 1 | 14.3 | 0 | 0.0 | 7 | 17.5 | |
| Moderately differentiated | 21 | 65.6 | 4 | 57.1 | 0 | 0.0 | 25 | 62.5 | |
| Poorly differentiated | 4 | 12.5 | 2 | 28.6 | 1 | 100.0 | 7 | 17.5 | |
| Missing | 1 | 3.1 | 0 | 0.0 | 0 | 0.0 | 1 | 2.5 | |
| Fuel used for heating and cooking | |||||||||
| Coal only | 29 | 90.6 | 4 | 57.1 | 0 | 0.0 | 33 | 82.5 | |
| Coal and wood | 1 | 3.1 | 0 | 0.0 | 0 | 0.0 | 1 | 2.5 | |
| Electricity | 0 | 0.0 | 2 | 28.6 | 0 | 0.0 | 2 | 5.0 | |
| Missing | 2 | 6.3 | 1 | 14.3 | 1 | 100.0 | 4 | 10.0 | |
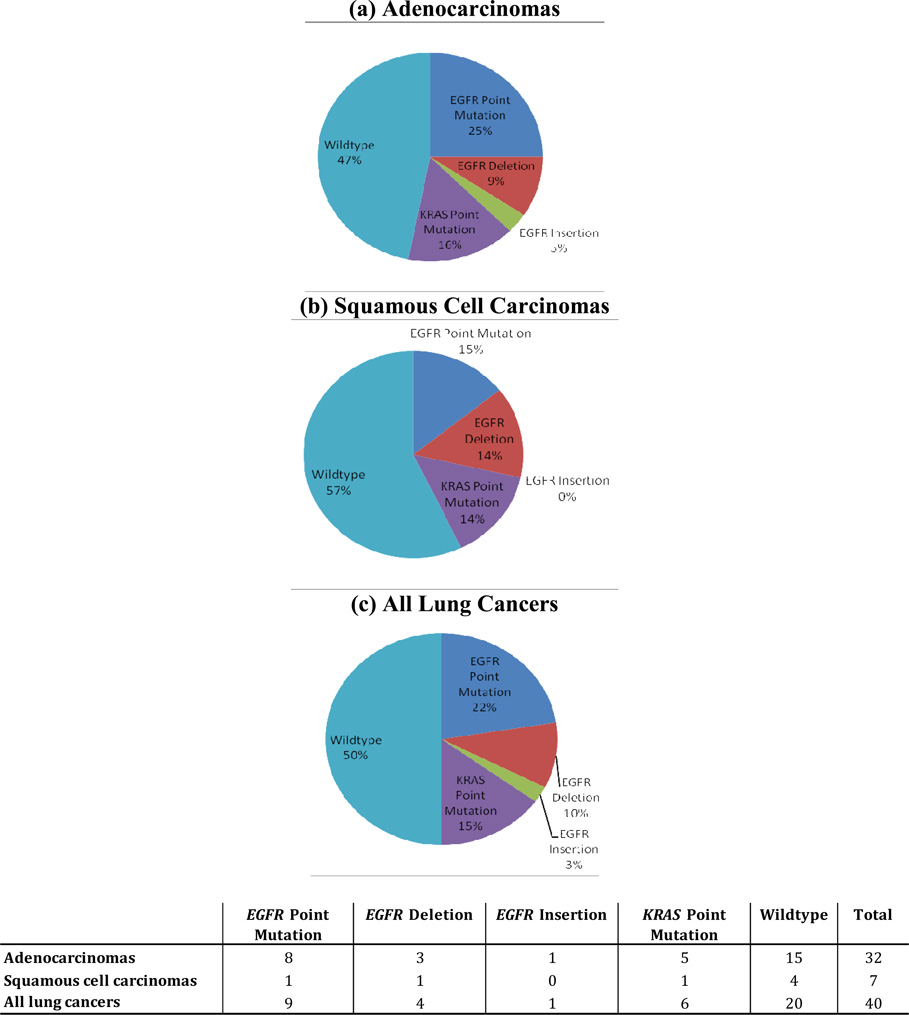
Mutations in tumor tissues were observed only in EGFR and KRAS. No mutations were detected for BRAF, NRAS, PIK3CA, MEK1, HER2, AKT1, or PTEN. KRAS mutations were found in 15% of all tissues, and EGFR mutations were found in 35% of all tissues (Figure 1). When stratifying the EGFR mutations by type, point mutations were found in 22% of all tissues, deletions in 10%, and insertions in 3% (Figure 1). All EGFR and KRAS mutations were mutually exclusive. While the distribution of EGFR mutations suggests the potential for variability by age, the distributions were fairly similar by histology and differentiation (Table 2).
Figure 1.
EGFR point mutations, insertions, and deletions, and KRAS point mutations detected in never smoking female (a) adenocarcinomas, (b) squamous cell carcinomas, and (c) all lung cancers†
† EGFR and KRAS point mutations, insertions, and deletions were mutually exclusive; One tissue had two unique EGFR point mutations; All lung cancers includes the adenosquamous cell carcinoma case in addition to the adenocarinomas and squamous cell carcinomas
Table 2.
Assocations between clinicopathologic characteristics and EGFR and KRAS events among never smoking female lung cancer patients†
| Wildtype | EGFR point mutations, deletions, and insertions | KRAS point mutations | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| absent | present | absent | present | ||||||||||||
| n | % | n | % | n | % | P-value†† | P-value††† | n | % | n | % | P-value†† | P-value††† | ||
| Age | 0.22 | 0.21 | 1.00 | 0.73 | |||||||||||
| <50 years old | 11 | 57.9 | 14 | 56.0 | 5 | 35.7 | 16 | 48.5 | 3 | 50.0 | |||||
| ≥50 years old | 8 | 42.1 | 11 | 44.0 | 9 | 64.3 | 17 | 51.5 | 3 | 50.0 | |||||
| Histology | 0.66 | 0.62 | 0.0 | 1.00 | 0.81 | ||||||||||
| Adenocarcinoma | 15 | 78.9 | 20 | 80.0 | 12 | 85.7 | 27 | 81.8 | 5 | 83.3 | |||||
| Squamous Cell Carcinoma | 4 | 21.1 | 5 | 20.0 | 2 | 14.3 | 6 | 18.2 | 1 | 16.7 | |||||
| Differentiation | 0.92 | 0.98 | 0.44 | 0.43 | |||||||||||
| Well differentiated | 4 | 21.1 | 4 | 16.0 | 3 | 21.4 | 7 | 21.2 | 0 | 0.0 | |||||
| Moderately differentiated | 11 | 57.9 | 16 | 64.0 | 9 | 64.3 | 20 | 60.6 | 5 | 83.3 | |||||
| Poorly differentiated | 3 | 15.8 | 4 | 16.0 | 2 | 14.3 | 5 | 15.2 | 1 | 16.7 | |||||
Restricted to adenocarcinomas and squamous cell carcinomas; Wildtype subjects are defined as having no EGFR point mutations, deletions, or insertions, and no KRAS point mutations; P-values determined by Chi-squared and Fischer's exact tests
P-value for EGFR point mutations, deletions, and insertions being present versus absent, or KRAS point mutations being present versus absent, respectively
P-value for EGFR point mutations, deletions, and insertions being present versus wildtype, or KRAS point mutations being present versus wildtype, respectively
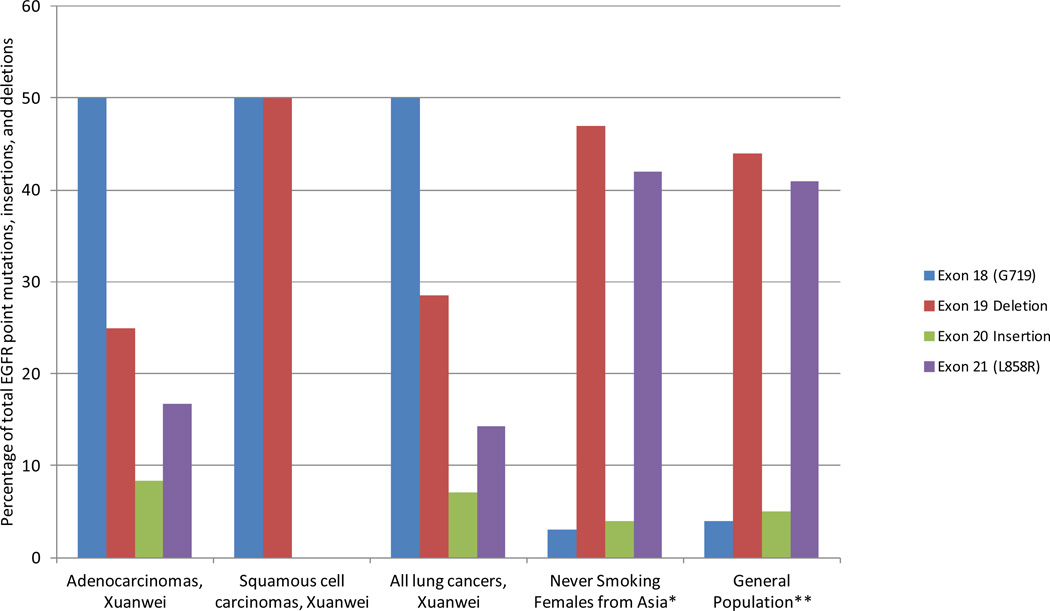
Among the 15 EGFR mutations, nine were point mutations, four were exon 19 deletions, and one was an exon 20 insertion (Table 3). Eight point mutations were in adenocarcinomas, with one tissue having two different EGFR point mutations, and one mutation was in a squamous cell carcinoma. EGFR point mutations were predominately G719 mutations (n = 7, or 46% of all EGFR mutations) on exon 18 (Figure 2). EGFR point mutations were also observed on exon 21 (L858R, n = 2, or 13% of all EGFR mutations). Three adenocarcinomas and one squamous cell carcinoma harbored exon 19 deletions, and one adenocarcinoma contained an exon 20 insertion. Among the six KRAS mutations, all were KRAS G12C_34G>T point mutations (Table 3). Five of these mutations were found in adenocarcinomas and 1 was in a squamous cell carcinoma.
Table 3.
EGFR and KRAS point mutations, deletions, and insertions in never smoking lung cancer tumors
| Gene | Event | Description | Histology | Differentiation | Age | Fuel Used for Heating and Cooking** |
|---|---|---|---|---|---|---|
| EGFR | ||||||
| Point Mutation | ||||||
| EGFR_G719A_2156G>C* | Adenocarcinoma | Well | 40 | Only Coal | ||
| EGFR_L858R_2573T>G | Adenocarcinoma | Well | 40 | Only Coal | ||
| EGFR_G719C_2155G>T | Adenocarcinoma | Well | 56 | Only Coal | ||
| EGFR_G719A_2156G>C | Adenocarcinoma | Moderately | 52 | Only Coal | ||
| EGFR_G719C_2155G>T | Adenocarcinoma | Moderately | 52 | Only Coal | ||
| EGFR_L858R_2573T>G | Adenocarcinoma | Moderately | 58 | Only Coal | ||
| EGFR_G719S _2155G>T | Adenocarcinoma | Poorly | 30 | Only Coal | ||
| EGFR_G719S_2155G>A | Adenocarcinoma | Poorly | 40 | Only Coal | ||
| EGFR_G719C_2155G>T | Squamous Cell Carcinoma | Moderately | 58 | Only Coal | ||
| Deletion | ||||||
| EGFR_19-15bpDel | Adenocarcinoma | Moderately | 43 | Only Coal | ||
| EGFR_19-15bpDel | Adenocarcinoma | Moderately | 52 | Unknown | ||
| EGFR_19-15bpDel | Adenocarcinoma | Moderately | 58 | Coal and Wood | ||
| EGFR_19-15bpDel | Squamous Cell Carcinoma | Moderately | 50 | Electric | ||
| Insertion | ||||||
| E20-6bp-ins. | Adenocarcinoma | Moderately | 50 | Only Coal | ||
| KRAS | ||||||
| Point Mutation | ||||||
| KRAS(R)G12C_34G>T | Adenocarcinoma | Moderately | 30 | Only Coal | ||
| KRAS(R)G12C_34G>T | Adenocarcinoma | Moderately | 40 | Only Coal | ||
| KRAS(R)G12C_34G>T | Adenocarcinoma | Moderately | 50 | Only Coal | ||
| KRAS(R)G12C_34G>T | Adenocarcinoma | Moderately | 52 | Only Coal | ||
| KRAS(R)G12C_34G>T | Adenocarcinoma | Moderately | 52 | Only Coal | ||
| KRAS(R)G12C_34G>T | Squamous Cell Carcinoma | Poorly | 24 | Only Coal |
This tumor also had a second EGFR point mutation [EGFR(R)L861Q_2582T>A]
Type of fuel currently used at the time of surgery
Figure 2.
Distribution of EGFR exon 18 point mutations, exon 19 deletions, exon 20 insertions, and exon 21 point mutations in never smoking female adenocarcinomas (n = 32), squamous cell carcinomas (n = 7), and all lung cancers (n = 40) from Xuanwei, China†.
† One tissue had two unique EGFR point mutations; Total number of EGFR point mutations, deletions, and insertions present in the Xuanwei samples is 12 among adenocarcinomas, 2 among squamous cell carcinomas, and 14 among all lung cancers; * Mutation rates among never smoking females in Asia reported by (Zhang et al 2012); ** Mutation rates typically found when summarizing all lung cancer histologies, across multiple ethnicities including both those that do and do not smoke tobacco, reported by (Shigematsu and Gazdar 2006).
EGFR and KRAS mutations were observed in both adenocarcinomas and squamous cell carcinomas and for a range of differentiations and ages (Table 3). One consistency observed for all point mutations was the use of only coal for the case's household cooking and heating needs, which may not be surprising given that most patients were coal-only users. This consistency was not observed for EGFR deletions. No significant differences were observed when comparing mutation distributions by coal type: bituminous coal (n = 21) versus anthracite coal (n = 12) (p >0.05).
Discussion
We have conducted, to the best of our knowledge, the first analysis of point mutations, insertions, and deletions in a spectra of known lung cancer driver genes that includes genes beyond EGFR and KRAS, in tumor tissues from a population of never smokers who used solid fuel in their home for heating and cooking. When compared to previously studied populations, our results suggest a unique distribution of EGFR and KRAS mutations. Specifically, our observed point mutation frequencies of EGFR exons 18 and 21, suggest a unique mutational pattern in lung cancer tumors for subjects with substantial indoor air pollution exposure from coal burning.
Our assay evaluated the following EGFR point mutations: G719: p.G719C c.2155G_T, p.G719S c.2155G_A, p.G719A c.2156G_C; T790M: p.T790M c.2369C_T; L858: p.L858R c.2573T_G; L861: p.L861Q c.2582T_A. Similar to other studies in never smokers [18, 19, 21], we found a lack of association between clinicopathological characteristics and EGFR mutations. In terms of overall EGFR mutation frequencies, our results are also consistent with previous reports that about 30% of lung cancer tumors from Eastern Asia harbor EGFR mutations, and about 40% of lung cancer tumors from females harbor EGFR mutations [22]. When summarizing all lung cancer histologies, across multiple ethnicities including both those that do and do not smoke tobacco, EGFR mutations are mostly exon 19 deletions (44%) and exon 21 L858R point mutations (41%), with only limited exon 20 insertions (5%) and exon 18 G719 point mutations (4%) [22]. Our observations are fairly consistent with the findings from characterizations of tissues from never smoking females both in East Asia and in North America, with respect to the percentage of EGFR mutations that are exon 19 deletions [15, 18, 19, 21, 23] and exon 20 insertions [21].
In contrast, a striking difference exists between our findings and those typically found for the incidence of G719 mutations in exon 18 (50% versus 4%) and L858 mutations in exon 21 (14% versus 41%). Interestingly, a recent characterization of lung adenocarcinomas from never smoking females in Shanghai [15] found virtually identical mutation rates for exon 18 (3%) and exon 21 mutations (42%) as those typically found across ethnicities when including both those that do and do not smoke tobacco [22]. When taken together, these results suggest that not only is our high incidence of G719 mutations in exon 18 and unusually low incidence of L858 mutations in exon 21 different from the general population of all lung cancer tumors, it is also divergent from tumors collected from other never smoking female populations in China. Indeed, the distribution of mutations we observed among all lung cancers was significantly different than those previously observed in the general population [22] (p < 0.0001) and in a series of never smoking females from Asia [21] (p < 0.0001) (Figure 2). There was no statistical difference between the mutation distributions of these two comparative, previously reported populations (p > 0.05). Of note, PCR carryover of the EGFR exon 18 mutation is unlikely to explain our results, as no template controls are used throughout our assay [20]. Recently, the TCGA study of 178 squamous cell lung cancer samples found no EGFR L858R or exon 19 deletions [24], further highlighting the uniqueness of our study population and findings.
The high percentage of samples with KRAS mutations (15%) in our series is also of interest primarily because KRAS mutations are reportedly more rare in other populations from Asia (~5%) [25, 26] and populations of never smokers from Asia (~2%) [15, 18]. Our findings are consistent with a previous analysis of nonsmoking women in this region of the world, which found that 22% of the lung tumors or sputum samples from Xuanwei harbored a KRAS mutation compared to 7% of samples from urban Beijing [27]. An additional study carried out in Xuanwei tumors found the frequency of KRAS mutations to be slightly higher (29%) [28]. Both of the previous studies [27, 28], as well as the current report, found a majority (>66%) of the KRAS mutations to be G to T.
Given that our population was from the a rural region of the Yunnan Province, whereas the populations used for previous characterizations of never smoking female lung tumors were selected from urban Shanghai and Beijing, our divergent findings for KRAS mutations and EGFR mutations on exons 18 and 21 may be due to the varying environmental exposures in urban versus rural China. The main difference in environmental exposures is likely indoor air pollution associated with in-home solid fuel use. The primary source of indoor air pollution in Xuanwei is smoke from domestic fuel combustion for heating and cooking, with most residents burning bituminous coal and some anthracite coal and wood. Xuanwei residents, and more specifically the women who primarily do all of the cooking, experience substantial exposures to indoor air pollution attributed to coal burning [29]. In Shanghai and Beijing, most residents typically use modern fuel sources, such as natural gas or electricity, for their heating and cooking needs.
Coal combustion for heating and cooking increases the levels in the home of known carcinogens, such as polycyclic aromatic hydrocarbons (PAHs) [30]. Although more research is needed to determine which coal constituent(s) is driving the risk of lung cancer associated with household coal use, it is conceivable that there may be mutation patterns in lung cancer tumors that will vary based on not only smoking status, but also these environmental exposures experienced by the patient. In support of our hypothesis, PAHs have been shown to increase intracellular calcium in human cell lines [31], which may lead to EGFR-dependant cell proliferation [32, 33]. PAH-DNA adducts have also been observed in bronchoalveolar lavage in Xuanwei residents burning coal [34], suggesting that the lung tumors in our never smoking tissues are being induced by coal combustion byproducts, such as PAHs, potentially leading to unique mutational patterns. Further, mutations in the p53 gene in tumor samples from nonsmoking women in Xuanwei were consistent with those of PAHs and different from those of lung cancer tumors from smokers [28]. This previous study in Xuanwei found that 76% of the mutations in TP53 were G > T and that 33% of all the mutations clustered at a GC-rich region, which is a preferred binding site for PAHs. Additional evidence for the role of PAHs in the mutation spectrum was demonstrated by the fact that smoky coal condensate induced primarily G > T mutations at a frequency (78–86%) that was similar to that of B[a]P (77%) in Salmonella [35]. Since most of the mutations observed in our study were G to T transversions and some G to C transversions, which are rarely observed in other populations, our findings suggest that coal-derived carcinogens (i.e., PAHs) are the etiologic agent in this population.
In conclusion, the tissues from our never smoking female population harbored a strikingly divergent mutational pattern for EGFR and KRAS, specifically with respect to EGFR exon 18, EGFR exon 21, compared to other never smoking female populations in Asia. Our findings have helped evaluate the impact coal combustion byproducts have on the lung, and provide new insights into the pathogenesis of lung cancer among never smoking females exposed to indoor air pollution from coal. We found that mutations in KRAS and EGFR were mutually exclusive, potentially suggesting differing carcinogenic pathways; however, additional research is needed to determine if these mutations are indeed driver mutations or merely passenger mutations [36]. Due to our small sample size, caution should be used when drawing conclusions from our results, particularly from subset analyses, until these findings are replicated in a larger study. Our results can serve as a comparative model for the study of lung cancer tumors from never smoking females without this exposure, both in Asia and elsewhere, and also highlight the potential importance of integrating environmental exposure histories into clinical and translational research. In this vein, however, our results may only be relevant to similar populations, with similar histologies and grades of lung cancer. Efforts should be made to evaluate the driver mutation spectra of subjects who use additional household fuel sources beyond only coal, which was the case for most of our subjects. Future research is needed to integrate additional exposure data exploring coal use dose-response analyses in terms of duration, intensity, and amount of coal use and exposure to environmental tobacco smoke.
Acknowledgements
Funding: The Intramural National Cancer Institute (N01-CO-12400) program funded the work. WP was also funded through R01-CA121210.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None to declare.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer. 2007;7:778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 3.Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol. 2007;25:561–570. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 4.Lee YJ, Kim JH, Kim SK, Ha SJ, Mok TS, et al. Lung cancer in never smokers: change of a mindset in the molecular era. Lung Cancer. 2011;72:9–15. doi: 10.1016/j.lungcan.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Lam WK. Lung cancer in Asian women-the environment and genes. Respirology. 2005;10:408–417. doi: 10.1111/j.1440-1843.2005.00723.x. [DOI] [PubMed] [Google Scholar]
- 6.Mumford JL, He XZ, Chapman RS, Cao SR, Harris DB, et al. Lung cancer and indoor air pollution in Xuan Wei, China. Science. 1987;235:217–220. doi: 10.1126/science.3798109. [DOI] [PubMed] [Google Scholar]
- 7.Hosgood HD, Boffetta P, III, Greenland S, Lee Y-CA, McLaughlin J, et al. In-Home Coal and Wood Use and Lung Cancer Risk: A Pooled Analysis of the International Lung Cancer Consortium. Environ Health Perspect. 2010;118:1743–1747. doi: 10.1289/ehp.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lan Q, He XZ. Molecular epidemiological studies on the relationship between indoor coal burning and lung cancer in Xuan Wei, China. Toxicology. 2004;198:301–305. doi: 10.1016/j.tox.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Chapman RS, Mumford JL, Harris DB, He ZZ, Jiang WZ, et al. The epidemiology of lung cancer in Xuan Wei, China: current progress, issues, and research strategies. Arch Environ Health. 1988;43:180–185. doi: 10.1080/00039896.1988.9935850. [DOI] [PubMed] [Google Scholar]
- 10.Lan Q, Chapman RS, Schreinemachers DM, Tian L, He X. Household stove improvement and risk of lung cancer in Xuanwei, China. J Natl Cancer Inst. 2002;94:826–835. doi: 10.1093/jnci/94.11.826. [DOI] [PubMed] [Google Scholar]
- 11.Hosgood HD, Chapman R, Shen M, Blair A, Chen E, et al. Portable stove use is associated with lower lung cancer mortality risk in lifetime smoky coal users. British Journal of Cancer. 2008;99:1934–1939. doi: 10.1038/sj.bjc.6604744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, et al. Carcinogenicity of household solid fuel combustion and of high-temperature frying. Lancet Oncol. 2006;7:977–978. doi: 10.1016/s1470-2045(06)70969-x. [DOI] [PubMed] [Google Scholar]
- 13.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. The Lancet Oncology. 2011;12:175–180. doi: 10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]
- 14.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, et al. Clinical and Biological Features Associated With Epidermal Growth Factor Receptor Gene Mutations in Lung Cancers. Journal of the National Cancer Institute. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Ren Y, Fang Z, Li C, Fang R, et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol. 2010;28:4616–4620. doi: 10.1200/JCO.2010.29.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Fang R, Sun Y, Han X, Li F, et al. Spectrum of oncogenic driver mutations in lung adenocarcinomas from East Asian never smokers. PLoS ONE. 2011;6:e28204. doi: 10.1371/journal.pone.0028204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YJ, Cho BC, Jee SH, Moon JW, Kim SK, et al. Impact of Environmental Tobacco Smoke on the Incidence of Mutations in Epidermal Growth Factor Receptor Gene in Never-Smoker Patients With Non–Small-Cell Lung Cancer. Journal of Clinical Oncology. 2010;28:487–492. doi: 10.1200/JCO.2009.24.5480. [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi T, Ando M, Kubo A, Takada M, Atagi S, et al. Long Exposure of Environmental Tobacco Smoke Associated with Activating EGFR Mutations in Never-Smokers with Non–Small Cell Lung Cancer. Clinical Cancer Research. 2011;17:39–45. doi: 10.1158/1078-0432.CCR-10-1773. [DOI] [PubMed] [Google Scholar]
- 19.Taga M, Mechanic LE, Hagiwara N, Vahakangas KH, Bennett WP, et al. EGFR somatic mutations in lung tumors: radon exposure and passive smoking in former- and never-smoking U.S. women. Cancer Epidemiol Biomarkers Prev. 2012;21:988–992. doi: 10.1158/1055-9965.EPI-12-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su Z, Dias-Santagata D, Duke M, Hutchinson K, Lin YL, et al. A platform for rapid detection of multiple oncogenic mutations with relevance to targeted therapy in non-small-cell lung cancer. J Mol Diagn. 2011;13:74–84. doi: 10.1016/j.jmoldx.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Sun Y, Pan Y, Li C, Shen L, et al. Frequency of Driver Mutations in Lung Adenocarcinoma from Female Never-Smokers Varies with Histologic Subtypes and Age at Diagnosis. Clinical Cancer Research. 2012;18:1947–1953. doi: 10.1158/1078-0432.CCR-11-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer. 2006;118:257–262. doi: 10.1002/ijc.21496. [DOI] [PubMed] [Google Scholar]
- 23.Bae NC, Chae MH, Lee MH, Kim KM, Lee EB, et al. EGFR, ERBB2, and KRAS mutations in Korean non-small cell lung cancer patients. Cancer Genetics and Cytogenetics. 2007;173:107–113. doi: 10.1016/j.cancergencyto.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Cancer Genome Atlas Research N. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu CC, Hsu HY, Liu HP, Chang JW, Chen YT, et al. Reversed mutation rates of KRAS and EGFR genes in adenocarcinoma of the lung in Taiwan and their implications. Cancer. 2008;113:3199–3208. doi: 10.1002/cncr.23925. [DOI] [PubMed] [Google Scholar]
- 26.Koivunen JP, Kim J, Lee J, Rogers AM, Park JO, et al. Mutations in the LKB1 tumour suppressor are frequently detected in tumours from Caucasian but not Asian lung cancer patients. Br J Cancer. 2008;99:245–252. doi: 10.1038/sj.bjc.6604469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keohavong P, Lan Q, Gao WM, DeMarini DM, Mass MJ, et al. K-ras mutations in lung carcinomas from nonsmoking women exposed to unvented coal smoke in China. Lung Cancer. 2003;41:21–27. doi: 10.1016/s0169-5002(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 28.DeMarini DM, Landi S, Tian D, Hanley NM, Li X, et al. Lung tumor KRAS and TP53 mutations in nonsmokers reflect exposure to PAH-rich coal combustion emissions. Cancer Res. 2001;61:6679–6681. [PubMed] [Google Scholar]
- 29.Mumford JL, Li X, Hu F, Lu XB, Chuang JC. Human exposure and dosimetry of polycyclic aromatic hydrocarbons in urine from Xuan Wei, China with high lung cancer mortality associated with exposure to unvented coal smoke. Carcinogenesis. 1995;16:3031–3036. doi: 10.1093/carcin/16.12.3031. [DOI] [PubMed] [Google Scholar]
- 30.IARC. Polynuclear aromatic compounds; Humans IMotEotCRt, editor. Lyon: International Agency for Research on Cancer Monographs on the Evaluation of the Carcinogenic Risks to Humans; 1983. [Google Scholar]
- 31.Tannheimer SL, Barton SL, Ethier SP, Burchiel SW. Carcinogenic polycyclic aromatic hydrocarbons increase intracellular Ca2+ and cell proliferation in primary human mammary epithelial cells. Carcinogenesis. 1997;18:1177–1182. doi: 10.1093/carcin/18.6.1177. [DOI] [PubMed] [Google Scholar]
- 32.Ethier SP, Cundiff KC. Importance of Extended Growth Potential and Growth Factor Independence on in Vivo Neoplastic Potential of Primary Rat Mammary Carcinoma Cells. Cancer Research. 1987;47:5316–5322. [PubMed] [Google Scholar]
- 33.Ethier SP, Chiodino C, Jones RF. Role of Growth Factor Synthesis in the Acquisition of Insulin/Insulin-like Growth Factor I Independence in Rat Mammary Carcinoma Cells. Cancer Research. 1990;50:5351–5357. [PubMed] [Google Scholar]
- 34.Mumford JL, Lee XM, Lewtas J, Young TL, Santella RM. DNA Adducts as Biomarkers for Assessing Exposure to Polycyclic Aromatic-Hydrocarbons in Tissues from Xuan-Wei Women with High Exposure to Coal Combustion Emissions and High Lung-Cancer Mortality. Environmental Health Perspectives. 1993;99:83–87. doi: 10.1289/ehp.939983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Granville CA, Hanley NM, Mumford JL, DeMarini DM. Mutation spectra of smoky coal combustion emissions in Salmonella reflect the TP53 and KRAS mutations in lung tumors from smoky coal-exposed individuals. Mutat Res. 2003;525:77–83. doi: 10.1016/s0027-5107(02)00314-7. [DOI] [PubMed] [Google Scholar]
- 36.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, et al. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]