FIG. 3.
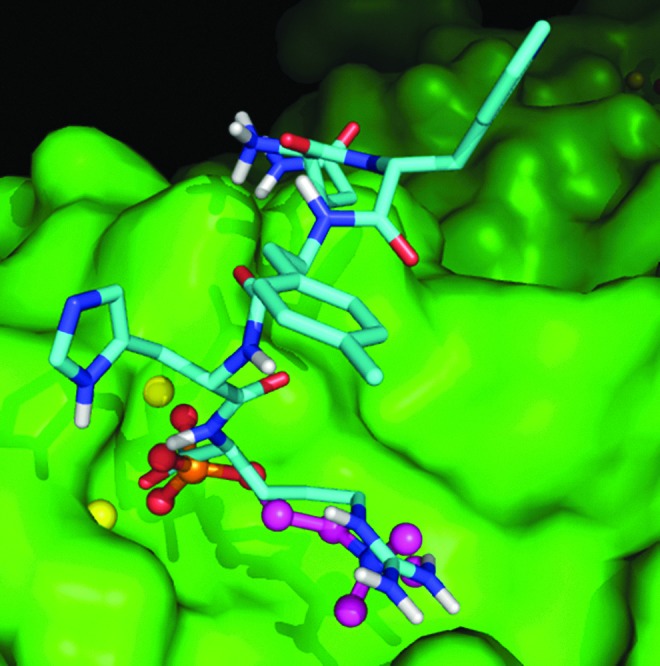
Molecular docking studies of CRPi-2 with C-reactive protein (CRP). Molecular docking studies were performed in the Lawrence Livermore National Laboratory as described in Materials and Methods. CRPi-2 interacts with the two Ca2+ ions (yellow spheres) in the single subunit of CRP. The carboxylate-terminus is coordinated to each metal ion. The Arg side chain of CRPi-2 (shown in cyan) is able to interact with the carboxylate side chain of Glu81. The binding of CRPi-2 is reminiscent of choline binding (shown as a ball-and-stick model). An additional interaction of the amide side chain of Asn and the amino-terminal amine coordinate to the carboxylate side chain of Glu147.
