Abstract
Trichomonads are among the earliest eukaryotes to diverge from the main line of eukaryotic descent. Keeping with their ancient nature, these facultative anaerobic protists lack two "hallmark" organelles found in most eukaryotes: mitochondria and peroxisomes. Trichomonads do, however, contain an unusual organelle involved in carbohydrate metabolism called the hydrogenosome. Like mitochondria, hydrogenosomes are double-membrane bounded organelles that produce ATP using pyruvate as the primary substrate. Hydrogenosomes are, however, markedly different from mitochondria as they lack DNA, cytochromes and the citric acid cycle. Instead, they contain enzymes typically found in anaerobic bacteria and are capable of producing molecular hydrogen. We show here that hydrogenosomes contain heat shock proteins, Hsp70, Hsp60, and Hsp10, with signature sequences that are conserved only in mitochondrial and alpha-Gram-negative purple bacterial Hsps. Biochemical analysis of hydrogenosomal Hsp60 shows that the mature protein isolated from the organelle lacks a short, N-terminal sequence, similar to that observed for most nuclear-encoded mitochondrial matrix proteins. Moreover, phylogenetic analyses of hydrogenosomal Hsp70, Hsp60, and Hsp10 show that these proteins branch within a monophyletic group composed exclusively of mitochondrial homologues. These data establish that mitochondria and hydrogenosomes have a common eubacterial ancestor and imply that the earliest-branching eukaryotes contained the endosymbiont that gave rise to mitochondria in higher eukaryotes.
Full text
PDF

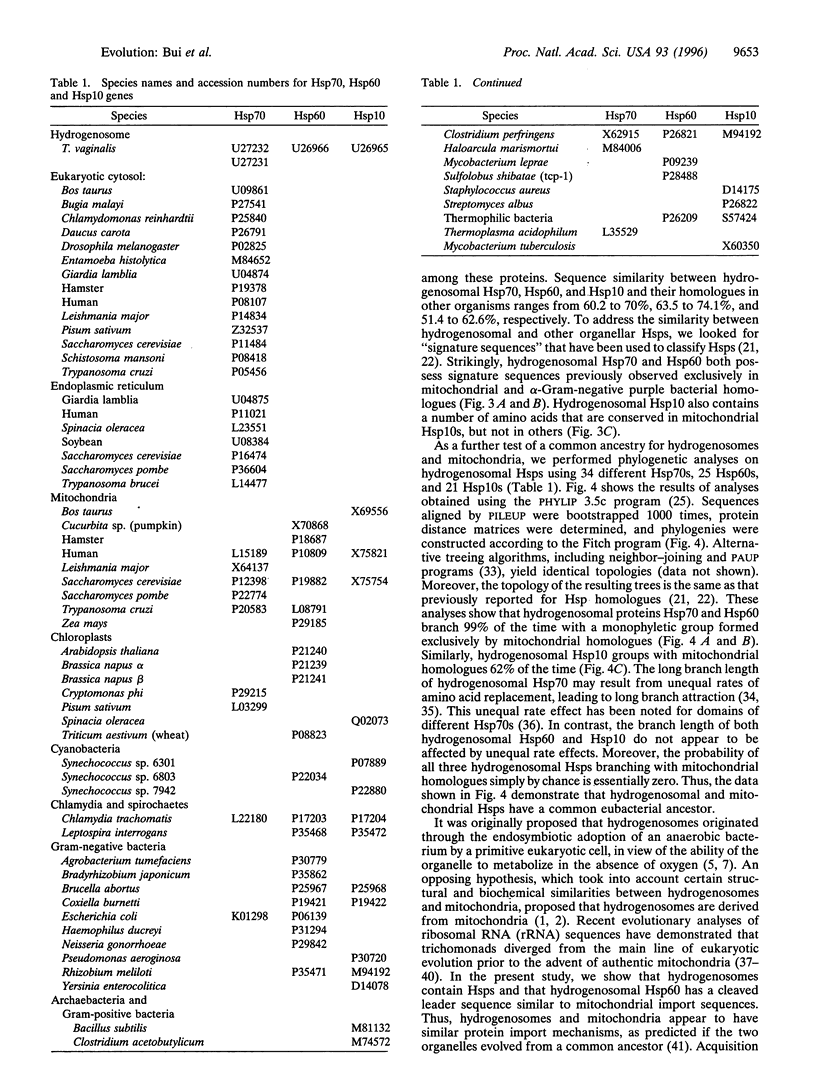
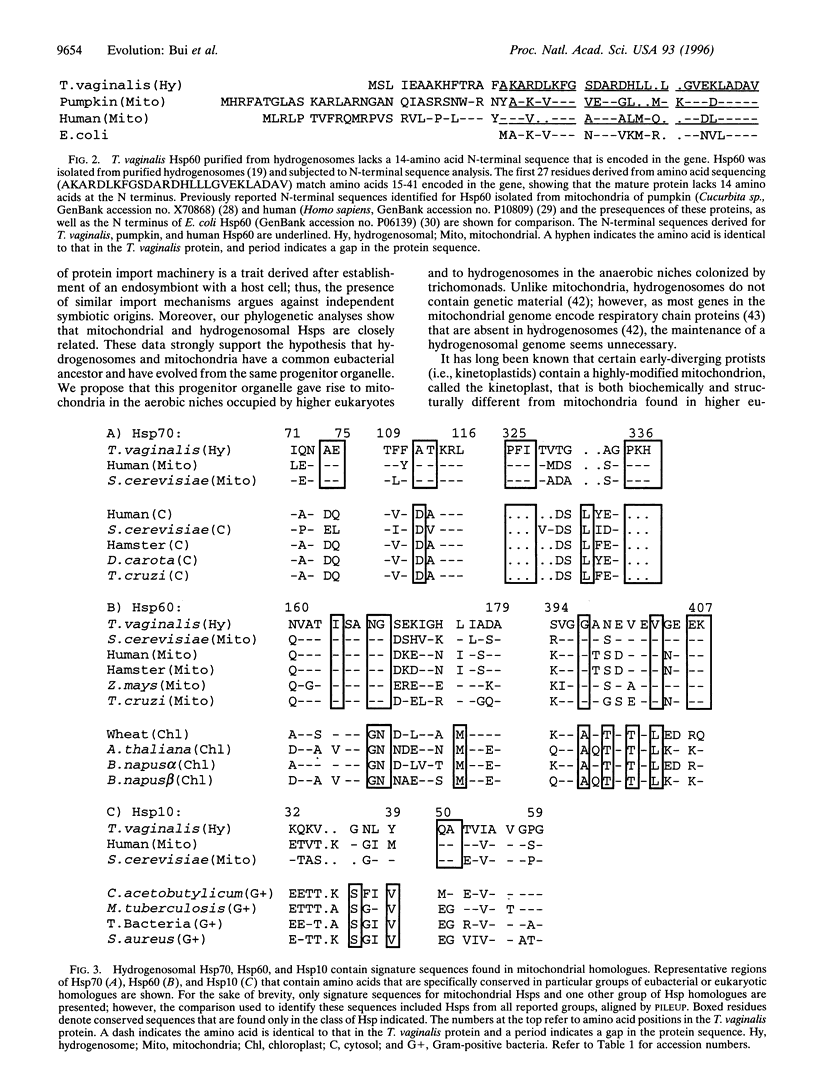
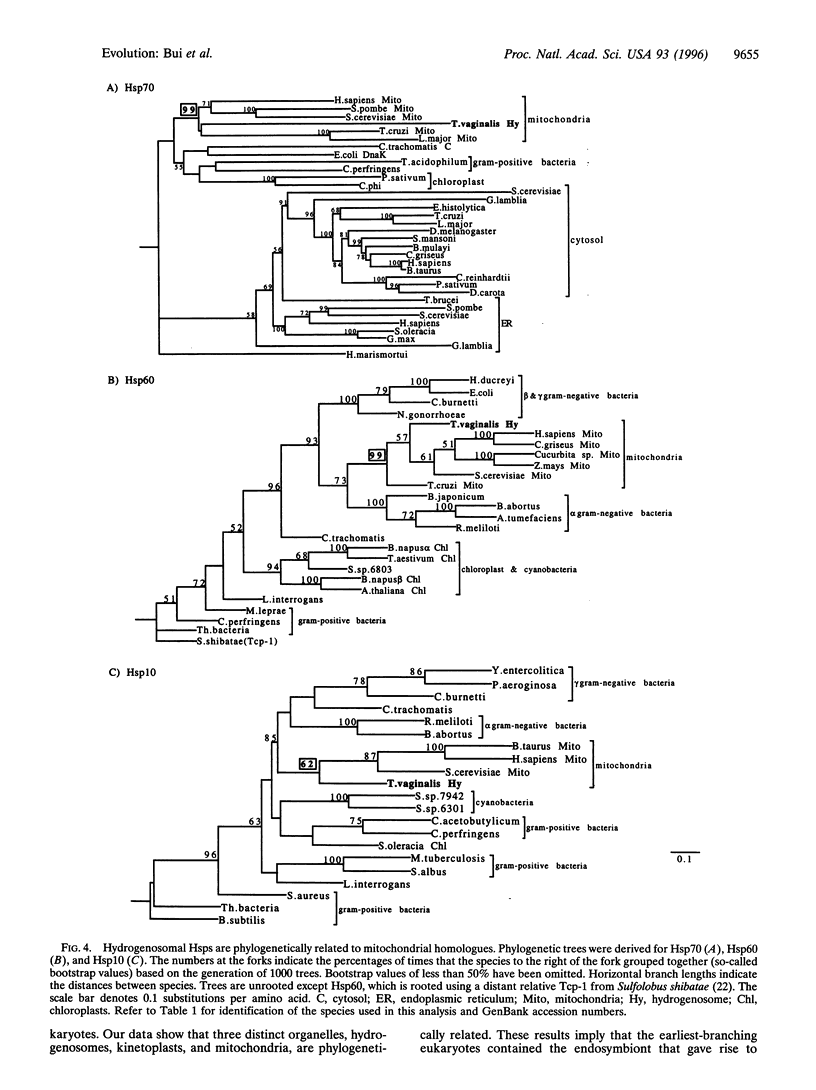

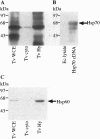
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- Baroin A., Perasso R., Qu L. H., Brugerolle G., Bachellerie J. P., Adoutte A. Partial phylogeny of the unicellular eukaryotes based on rapid sequencing of a portion of 28S ribosomal RNA. Proc Natl Acad Sci U S A. 1988 May;85(10):3474–3478. doi: 10.1073/pnas.85.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-Smith T. Eukaryotes with no mitochondria. 1987 Mar 26-Apr 1Nature. 326(6111):332–333. doi: 10.1038/326332a0. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. The number of symbiotic origins of organelles. Biosystems. 1992;28(1-3):91–108. doi: 10.1016/0303-2647(92)90011-m. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. The simultaneous symbiotic origin of mitochondria, chloroplasts, and microbodies. Ann N Y Acad Sci. 1987;503:55–71. doi: 10.1111/j.1749-6632.1987.tb40597.x. [DOI] [PubMed] [Google Scholar]
- Falah M., Gupta R. S. Cloning of the hsp70 (dnaK) genes from Rhizobium meliloti and Pseudomonas cepacia: phylogenetic analyses of mitochondrial origin based on a highly conserved protein sequence. J Bacteriol. 1994 Dec;176(24):7748–7753. doi: 10.1128/jb.176.24.7748-7753.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. S., Aitken K., Falah M., Singh B. Cloning of Giardia lamblia heat shock protein HSP70 homologs: implications regarding origin of eukaryotic cells and of endoplasmic reticulum. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2895–2899. doi: 10.1073/pnas.91.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. S. Evolution of the chaperonin families (Hsp60, Hsp10 and Tcp-1) of proteins and the origin of eukaryotic cells. Mol Microbiol. 1995 Jan;15(1):1–11. doi: 10.1111/j.1365-2958.1995.tb02216.x. [DOI] [PubMed] [Google Scholar]
- Honigberg B. M., Volkmann D., Entzeroth R., Scholtyseck E. A freeze-fracture electron microscope study of Trichomonas vaginalis Donné and Tritrichomonas foetus (Riedmüller). J Protozool. 1984 Feb;31(1):116–131. doi: 10.1111/j.1550-7408.1984.tb04300.x. [DOI] [PubMed] [Google Scholar]
- Hrdý I., Müller M. Primary structure and eubacterial relationships of the pyruvate:ferredoxin oxidoreductase of the amitochondriate eukaryote Trichomonas vaginalis. J Mol Evol. 1995 Sep;41(3):388–396. [PubMed] [Google Scholar]
- Hughes A. L. Nonlinear relationships among evolutionary rates identify regions of functional divergence in heat-shock protein 70 genes. Mol Biol Evol. 1993 Jan;10(1):243–255. doi: 10.1093/oxfordjournals.molbev.a039997. [DOI] [PubMed] [Google Scholar]
- Johnson P. J., d'Oliveira C. E., Gorrell T. E., Müller M. Molecular analysis of the hydrogenosomal ferredoxin of the anaerobic protist Trichomonas vaginalis. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6097–6101. doi: 10.1073/pnas.87.16.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandekar S. S., Bettencourt B. M., Kelley K. C., Recny M. A. A simple and rapid method for the purification of GroEL, an Escherichia coli homolog of the heat shock protein 60 family of molecular chaperonins. Protein Expr Purif. 1993 Dec;4(6):580–584. doi: 10.1006/prep.1993.1076. [DOI] [PubMed] [Google Scholar]
- Lahti C. J., Bradley P. J., Johnson P. J. Molecular characterization of the alpha-subunit of Trichomonas vaginalis hydrogenosomal succinyl CoA synthetase. Mol Biochem Parasitol. 1994 Aug;66(2):309–318. doi: 10.1016/0166-6851(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Lahti C. J., d'Oliveira C. E., Johnson P. J. Beta-succinyl-coenzyme A synthetase from Trichomonas vaginalis is a soluble hydrogenosomal protein with an amino-terminal sequence that resembles mitochondrial presequences. J Bacteriol. 1992 Nov;174(21):6822–6830. doi: 10.1128/jb.174.21.6822-6830.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J. A. Origin of the eukaryotic nucleus determined by rate-invariant analysis of rRNA sequences. Nature. 1988 Jan 14;331(6152):184–186. doi: 10.1038/331184a0. [DOI] [PubMed] [Google Scholar]
- Leipe D. D., Gunderson J. H., Nerad T. A., Sogin M. L. Small subunit ribosomal RNA+ of Hexamita inflata and the quest for the first branch in the eukaryotic tree. Mol Biochem Parasitol. 1993 May;59(1):41–48. doi: 10.1016/0166-6851(93)90005-i. [DOI] [PubMed] [Google Scholar]
- Lindmark D. G., Müller M. Hydrogenosome, a cytoplasmic organelle of the anaerobic flagellate Tritrichomonas foetus, and its role in pyruvate metabolism. J Biol Chem. 1973 Nov 25;248(22):7724–7728. [PubMed] [Google Scholar]
- Lloyd D., Hillman K., Yarlett N., Williams A. G. Hydrogen production by rumen holotrich protozoa: effects of oxygen and implications for metabolic control by in situ conditions. J Protozool. 1989 Mar-Apr;36(2):205–213. doi: 10.1111/j.1550-7408.1989.tb01075.x. [DOI] [PubMed] [Google Scholar]
- Lloyd D., Lindmark D. G., Müller M. Adenosine triphosphatase activity of Tritrichomonas foetus. J Gen Microbiol. 1979 Dec;115(2):301–307. doi: 10.1099/00221287-115-2-301. [DOI] [PubMed] [Google Scholar]
- Länge S., Rozario C., Müller M. Primary structure of the hydrogenosomal adenylate kinase of Trichomonas vaginalis and its phylogenetic relationships. Mol Biochem Parasitol. 1994 Aug;66(2):297–308. doi: 10.1016/0166-6851(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Müller M. Energy metabolism of protozoa without mitochondria. Annu Rev Microbiol. 1988;42:465–488. doi: 10.1146/annurev.mi.42.100188.002341. [DOI] [PubMed] [Google Scholar]
- Müller M. The hydrogenosome. J Gen Microbiol. 1993 Dec;139(12):2879–2889. doi: 10.1099/00221287-139-12-2879. [DOI] [PubMed] [Google Scholar]
- O'Fallon J. V., Wright R. W., Jr, Calza R. E. Glucose metabolic pathways in the anaerobic rumen fungus Neocallimastix frontalis EB188. Biochem J. 1991 Mar 1;274(Pt 2):595–599. doi: 10.1042/bj2740595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltauf F., Meingassner J. G. The absence of cardiolipin in hydrogenosomes of Trichomonas vaginalis and Tritrichomonas foetus. J Parasitol. 1982 Oct;68(5):949–950. [PubMed] [Google Scholar]
- Paul R. G., Williams A. G., Butler R. D. Hydrogenosomes in the rumen entodiniomorphid ciliate Polyplastron multivesiculatum. J Gen Microbiol. 1990 Oct;136(10):1981–1989. doi: 10.1099/00221287-136-10-1981. [DOI] [PubMed] [Google Scholar]
- Singh B., Patel H. V., Ridley R. G., Freeman K. B., Gupta R. S. Mitochondrial import of the human chaperonin (HSP60) protein. Biochem Biophys Res Commun. 1990 Jun 15;169(2):391–396. doi: 10.1016/0006-291x(90)90344-m. [DOI] [PubMed] [Google Scholar]
- Sogin M. L. Early evolution and the origin of eukaryotes. Curr Opin Genet Dev. 1991 Dec;1(4):457–463. doi: 10.1016/s0959-437x(05)80192-3. [DOI] [PubMed] [Google Scholar]
- Tsugeki R., Mori H., Nishimura M. Purification, cDNA cloning and Northern-blot analysis of mitochondrial chaperonin 60 from pumpkin cotyledons. Eur J Biochem. 1992 Oct 1;209(1):453–458. doi: 10.1111/j.1432-1033.1992.tb17309.x. [DOI] [PubMed] [Google Scholar]
- Viale A. M., Arakaki A. K., Soncini F. C., Ferreyra R. G. Evolutionary relationships among eubacterial groups as inferred from GroEL (chaperonin) sequence comparisons. Int J Syst Bacteriol. 1994 Jul;44(3):527–533. doi: 10.1099/00207713-44-3-527. [DOI] [PubMed] [Google Scholar]
- Viscogliosi E., Philippe H., Baroin A., Perasso R., Brugerolle G. Phylogeny of trichomonads based on partial sequences of large subunit rRNA and on cladistic analysis of morphological data. J Eukaryot Microbiol. 1993 Jul-Aug;40(4):411–421. doi: 10.1111/j.1550-7408.1993.tb04935.x. [DOI] [PubMed] [Google Scholar]
- Waldinger D., Eckerskorn C., Lottspeich F., Cleve H. Amino-acid sequence homology of a polymorphic cellular protein from human lymphocytes and the chaperonins from Escherichia coli (groEL) and chloroplasts (Rubisco-binding protein). Biol Chem Hoppe Seyler. 1988 Oct;369(10):1185–1189. doi: 10.1515/bchm3.1988.369.2.1185. [DOI] [PubMed] [Google Scholar]
- Whatley J. M., John P., Whatley F. R. From extracellular to intracellular: the establishment of mitochondria and chloroplasts. Proc R Soc Lond B Biol Sci. 1979 Apr 11;204(1155):165–187. doi: 10.1098/rspb.1979.0020. [DOI] [PubMed] [Google Scholar]
- Yarlett N., Hann A. C., Lloyd D., Williams A. Hydrogenosomes in the rumen protozoon Dasytricha ruminantium Schuberg. Biochem J. 1981 Nov 15;200(2):365–372. doi: 10.1042/bj2000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarlett N., Orpin C. G., Munn E. A., Yarlett N. C., Greenwood C. A. Hydrogenosomes in the rumen fungus Neocallimastix patriciarum. Biochem J. 1986 Jun 15;236(3):729–739. doi: 10.1042/bj2360729. [DOI] [PMC free article] [PubMed] [Google Scholar]