Table 1.
Recommended vaccinations for hematopoietic stem cell transplantation (SCT) recipients by the Korean Society of Infectious Diseases (KSID)
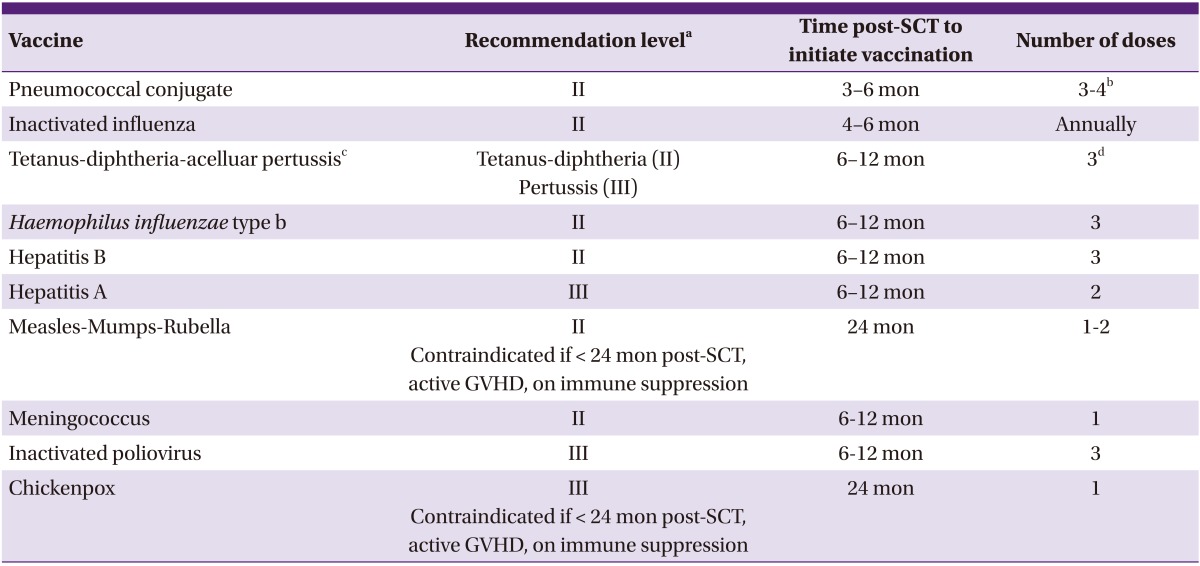
DTaP, diphtheria-tetanus-reduced acellular pertussis vaccine; GVHD, graft versus host disease; PCV, pneumococcal conjugate vaccine; SCT, hematopoietic stem cell transplantation; Td; tetanus toxoid-reduced diphtheria toxoid vaccine; Tdap, tetanus toxoid-reduced diphtheria toxoid-reduced acellular pertussis vaccine.
aStrength of recommendation: (I) Very strongly recommended: immunization may reduce mortality and be cost-effective. Most countries recommend the vaccination. (II) Strongly recommended: immunization may reduce mortality, but cost-effectiveness is unknown in Korea. Most developed countries recommend the vaccination. (III) Recommended: immunization may reduce morbidity rather than mortality. Cost-effectiveness is unknown. (U) Recommended reserved: lack of evidence for recommendation.
bFollowing the three doses of PCV, a dose of 23-valent polysaccharide pneumococcal vaccine may be given to broaden the covered spectrum (II). In SCT recipients with chronic GVHD who are likely to respond poorly to polysaccharide vaccine, a fourth PCV should be considered (III).
cDTaP is preferred over Tdap. If only Tdap is available, it can be used.
dRe-immunization with Td or Tdap at least every 10 years.
