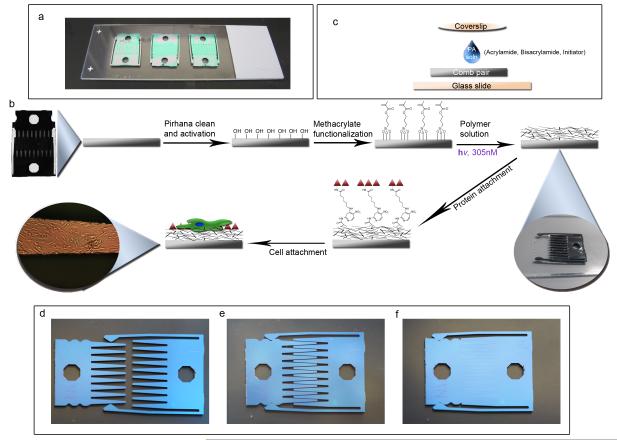
Figure 1. Setup and scheme of device modification.
(a) Three cleaned and methacrylate functionalized combs are placed into contact onto a DCDMS coated glass slide to demonstrate the scale of the device. (b) The overall scheme of the device begins with pirhana cleaning and UVO treatment to first activate the device surface. Next, methacrylate groups are functionalized on the surface in order to covalently link polyacrylamide to the surface. The polyacrylamide solution consists of acrylamide monomer, bisacrylamide crosslinker, water, and the azo-photoinitiatior. These are degassed, well mixed and added to the comb as shown in (c). 7.5 μL of polyacrylamide (PA) solution is placed on the comb on the DCDMS coated glass slide. A DCDMS coated coverslip is carefully added to the top of the drop of PA solution to allow even solution spreading over the device. After 10 minutes of exposure to 305 nm UV light, the PA has gelled and the coverslip is carefully peeled off (See inset). The combs are separated and placed in PBS to swell. Then sulfo-SANPAH crosslinker in HEPES buffer is added for 10 minutes under 350 nm UV light. The protein is attached and the device is sterilized with UV. Cells are then added to the surface. The final inset shows a finger of a comb containing ASC’s illuminated by a 40x upright microscope.