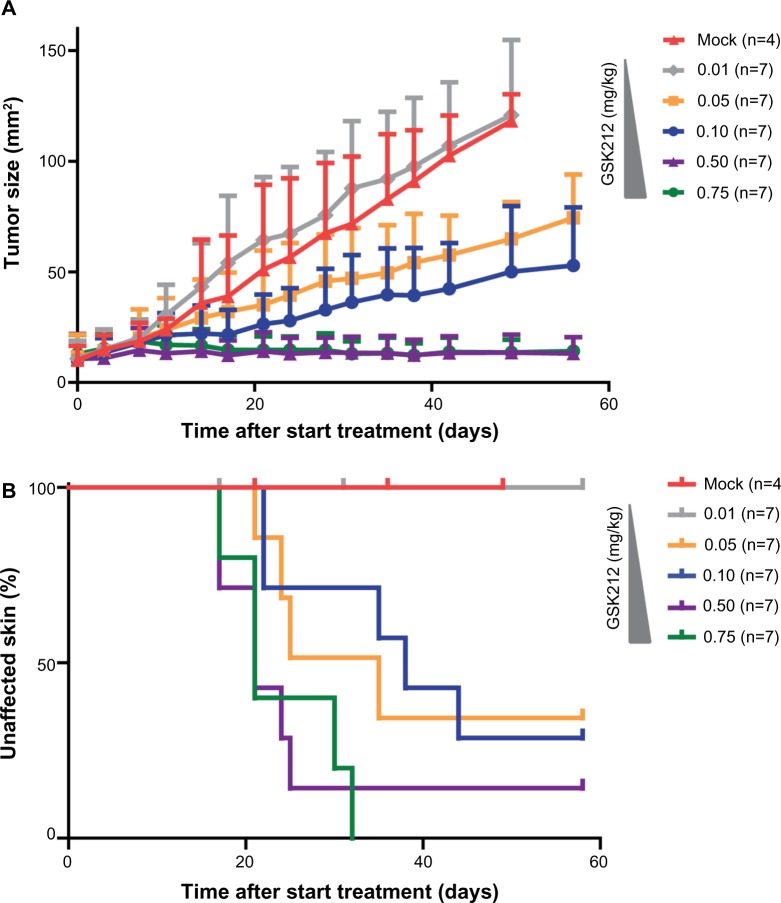
Figure 3.
MEKi dose reduction does not achieve reduced skin toxicity without impairing tumor control.
Notes: 4–10 week old BrafV600E/+/Pten−/− mice bearing on average 10 mm2 melanomas were placed on 0.01 (gray line), 0.05 (orange line), 0.1 (blue line), 0.5 (purple line), or 0.75 (green line) mg/kg GSK212 drug treatment. subsequently, the tumor outgrowth was followed over time and compared to mock-treated mice (red line) (A). For all cohorts of mice, the occurrence of skin toxicity was evaluated using a Kaplan–Meier analysis (B).
Abbreviations: MEK, mitogen-activated protein kinase kinase; MEKi, MEK inhibitor; BRAFi, BRAF inhibitor; GSK212, GSK1120212.