Abstract
Medical therapy of patients with malignancy requires a paradigm shift through development of new drugs with a good safety record and novel mechanisms of activity. While there is no dearth of such molecules, one particular agent, “reovirus” is promising by its ability to target cancer cells with aberrant signaling pathways. This double stranded RNA virus has been therapeutically formulated and has rapidly progressed from pre-clinical validation of anti cancer activity to a phase III registration study in platinum refractory metastatic squamous cell carcinoma of the head and neck. During this process, reovirus has demonstrated safety both as a single agent when administered intratumorally and intravenously, as well as in combination therapy, with multiple chemotherapeutics such as gemcitabine, carboplatin/paclitaxel, and docetaxel; and similarly with radiation. The scientific rationale for its development as an anticancer agent stems from the fact that it preferentially replicates in and induces lyses of cells with an activated Kras pathway. As documented in many previous studies, the initial observation of greater tropism in Kras compromised situation might certainly not be the sole and possibly not even the predominant reason for enhanced virulence. All the same, scientists have emphasized on Kras optimistically due to its high prevalence in various types of cancers. Incidence of Kras mutation has been found to be highest in pancreatic cancer (85–90%) followed by colorectal (35–45%) and lung (25–30%). Reovirus, in fact has the potential not only as a therapy but also as a tool to unravel the aberrant cellular pathway leading to carcinogenicity.
INTRODUCTION
Exciting research in the past decade has revealed unique viral characteristics not registered in any other micro-organism. Viruses harbor distinct strategies to overcome the sophisticated defense mechanisms of the infected host (1). The intricate ability to quickly gain control over the host cellular mechanism has potentially made the virus a doubled edged sword. While several viruses have been identified as cancer causing agents; many have also been identified as therapeutically viable oncolytic elements. One important member of therapeutically identified viruses is Respiratory Enteric Orphan virus commonly known as REOvirus (1). It is naturally oncolytic with inherent propensity to replicate in cells with dysfunctional cell signaling cascade including ras-activation. Preferential oncolytic properties of reovirus can be effectively exploited as clinical therapy due to general mildness of infection often requiring no special medical intervention.
The search for oncolytic viruses roots from the fact that transient cancer remission can occur following viral infections (2). Supportive preclinical evidence of the novel mechanisms of anticancer activity of reovirus provided the rationale for therapeutic advancement to clinical trials. Unique mode of tumor destruction encouraged oncolytic viral therapy as potential augmentation of chemotherapy and radiation thus making it a cutting edge clinical approach (3, 4).
Many of the oncolytic viruses currently in clinical testing are attenuated derivatives of prevalent human pathogens. Typically, in recent years, they have been genetically engineered to further reduce their pathogenicity, increase their oncolytic potency and enhance specificity for cancer tissue. Virus with a double stranded DNA genome is the most suitable candidate for such manipulations with greater genome stability and lesser chance of hazardous mutations. Adenoviruses and herpes simplex virus are the most suitable and thus been extensively engineered. Reverse genetic manipulation of RNA virus is still a scientific challenge even with the availability of the present day cutting edge genetic technology. In a similar vein, reovirus is not amenable to genetic engineering, especially due to its segmented structure of the dsRNA (5). However, considering the fact that this naturally oncolytic, and pathologically self resolving virus appears to be tumor cytotoxic, even in presence of neutralizing antibodies, the lack of engineerability may not be a constraint to its further therapeutic development.
PRE-CLINICAL STUDIES WITH REOVIRUS
Reovirus as a single agent
In vitro data with Reovirus
Several preclinical studies documented oncolytic characteristics of Reovirus (6, 7). Initial studies with NIH-3T3 cells revealed that resistance to reovirus infectivity can be overcome by transformation with activated Sos/Ras oncogenes (8, 9). The findings indicated that usurpation of Ras signaling pathway constitutes the basis of viral oncolysis (8). Several in vitro studies with human cancer cell lines documented the evident role of Reovirus in cellular cytopathy (4, 8). A systematic analysis of the propensity of reovirus infectivity towards 24 established glioma cell lines was conducted. Dramatic and widespread cell killing after exposure to live (but not dead) reovirus occurred in 20 (83%) cell lines. After 48 hours of infection, widespread cell death was found in U87, U251N, and A172 cell lines, and almost complete cell death was seen after 72 hours. In contrast, cells receiving either dead or no virus remained healthy. Furthermore, to ensure that cell lysis was due to viral replication, cells were reacted with rabbit anti-reovirus antibody, followed by FITC-conjugated goat anti-rabbit IgG when susceptible lines depicted the expression of the viral antigens. Replication of reovirus in susceptible lines was further confirmed by [35S]methionine metabolic labeling (4).
Short term primary culture from surgically excised human glioma have shown 100% sensitivity to reoviral oncolysis (4). Studies conducted to test the ability of reovirus to infect and kill primary cultures of brain tumors freshly excised from nine glioma patients intriguingly depicted complete growth arrest and cell death (100%), although similar level of reovirus mediated cellular cytopathy was not documented with seven primary meningioma cultures (4). The ability of reovirus to lyse all primary glioma cell cultures derived from surgical specimen suggested that a substantial proportion of gliomas may respond to reovirus treatment. It can be argued that the number of specimens being relatively small the observations might not reflect the comprehensive tumor properties of gliomas. The oncolytic spectrum of Reovirus has also been studied in 6 breast cancer cell lines where a high susceptibility was documented (10). An infectivity of 10 MOI (multiplicity of infection) was used in the study. Furthermore the control Hs578Bst normal mammary gland epithelial cell line used in the study did not show any cytopathic effect (CPE) to the virus confirming the fact that reovirus preferentially targets the transformed cells sparing the normal ones (9). Contemporary studies with melanoma cell lines and primary cultures from fresh resected tumors revealed similar results. The transformed cells allowed viral replication followed by caspase dependent cytotoxicity (11). Normal melanocytes conversely resisted viral replication. Notably, it was also reported that all of the screened breast cancer and melanoma cell lines had activated Kras.
The basis of the ability of reovirus to target and kill tumor cells but not to infect non-proliferating normal cells lies in its ability to usurp the highly activated signaling pathway found in tumor cells (4). This ability is most clearly established for Ras or elements in its downstream pathways. Ras activation is very common in malignant gliomas, colorectal (CRC) cancers as well as pancreatic malignancies.
Natural affinity of reovirus to kill and lyse cancer cells and the prevalence of Kras mutation in many of the studied models apparently instigated the researchers to attempt to draw a correlation between Kras mutation and viral oncolysis. It is to be noted that the fact is yet to be substantiated with scientific evidence. A somewhat similar situation was faced by the scientific community while developing the therapeutically viable adenovirus Onyx-01. The anti-tumor activity of the virus was initially proposed to be solely dependent on the status of p53 but deeper introspection revealed that lytic activity was observed in both p53 negative and WT conditions and the complex molecular mechanism of virus mediated oncolysis is yet to be clearly defined.(12).
Further studies to elucidate cellular events favoring reovirus mediated apoptosis confirmed that colon cancer cell lines, HEK293 and HCT116 displayed elevated beta-catenin expression to promote reovirus mediated oncolysis by down-regulation of NF-kappaB (3). Independent studies reported that reovirus activates human dendritic cells to promote innate antitumor immunity (13). The exact role of different immune effector cells in oncolytic virus mediated tumor regression has not yet been clearly defined. It is plausible that dendritic cells (DC) are likely to play a co-ordinating role in virus mediated immune response as key antigen presenting cells (APCs) that recognize the viral infection and regulate both innate and adaptive immunity (11). The fact that reovirus has been found to be effective in tumor cytopathy in spite of increasing neutralizing anti reovirus antibody (NARA) in the serum logically indicates the intricate role of the innate immune system in the virus mediated oncolytic process. The observation that reovirus activated DCs enhance the innate Natural Killer (NK) cells and cytotoxic T cells (Tc) by release of soluble factors inducing tumor cell killing via exocytosis clearly supports the hypothesis. The role of NK cells in tumor regression has been well documented in mouse model both by direct tumor recognition as well as via DC activation (14). Reovirus induced DC maturation also stimulated the production of proinflammatory cytokines IFN-alpha, TNF-alpha, IL-12p70, and IL-6. Activation of dendritic cells by reovirus was not dependent on viral replication, while cytokine production was inhibited by blockade of PKR (protein kinase receptor) and NF-kappaB signaling. These observations provide a hint that reovirus mediated DC activation and the downstream immune signaling is multimodal. Although systemic delivery activates the adaptive immune system and triggers a robust antibody response, intratumoral (ITu) injection of the virus results in successful tumor destruction by activation of the innate immune effector cells within the tumor micro-environment. Hence, reovirus recognition by dendritic cells may trigger innate effecter mechanisms to complement the virus's direct cytotoxicity, potentially enhancing the efficacy of reovirus as a therapeutic agent (13). Intravenous (IV) administration of reovirus in conjunction with immunosuppressants has been found to be therapeutically more effective indicating that the neutralizing antibodies are not completely blunted (15). All the same, the fact that antitumor activity is observed even in the presence of virus neutralizing antibodies can also indicate a plausible role of the host cellular machinery in camouflaging the virus and thus preventing its recognition by the specific antibodies. In a very elegantly conducted translational research study, it has been shown that replicating virus is detectable in cellular compartment of the blood, namely in mononuclear, granulocyte and platelets, and this may be a mechanism of protection from NARA (16). Furthermore, in this same clinical trial, when patients’ tumors were harvested, replicating virus was evident in tumor tissue but not from normal liver, demonstrating some evidence of selective tropism for malignant cells.
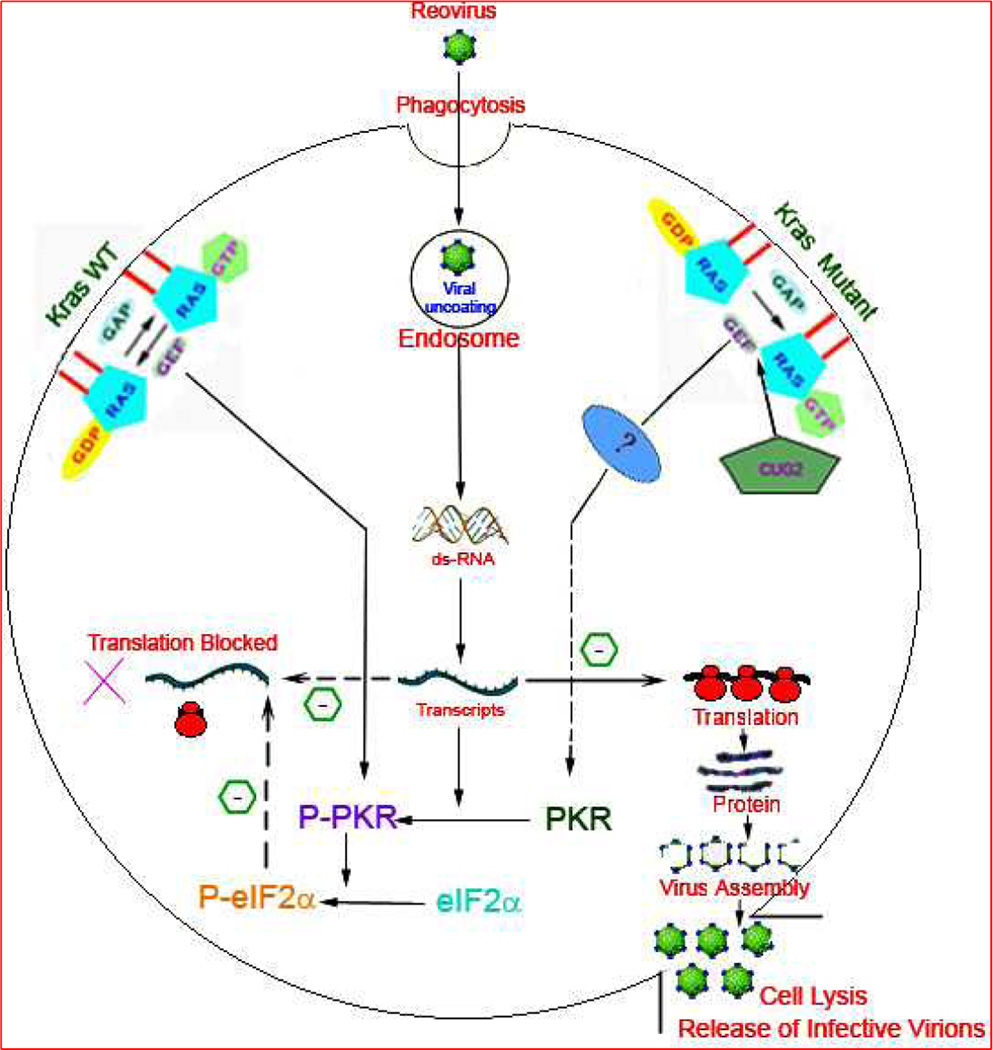
PKR plays crucial inhibitory role in efficient viral replication essential for infective virion production and oncolysis (7, 17)(Figure1). Expression of PKR is upregulated in response to INF released by infected cells (7). Binding to viral RNA/ initial transcripts results in PKR dimerization, autophosphorylation, and activation (7). The viral S1 segment mRNA has been shown to be potent activator of PKR (18). Once activated, PKR blocks the primary and secondary reovirus protein translation. In Ras transformed cells, PKR is not activated, allowing unabated viral replication and effective assembly of viral proteins for production of infection efficient virions (7, 17). Specific chemical inhibitors of PKR phosphorylation restore reovirus translation in untransformed cells providing evidence for a direct role of PKR in defining resistance to reovirus replication (9). Ras activation is suggested to release the translational blocks in the transformed cells. However, the exact molecular mechanism of co-ordination between Ras activation and inhibition of PKR mediated viral translational remains elusive.
Figure 1. A schematic illustration of the current hypothesis of the fate of reovirus upon entering the host cell by phagocytosis.
The status of the host cell signaling cascade determines the downstream consequences. K-ras (Kirsten rat sarcoma viral Oncogene) is a membrane bound molecular switch which binds to and catalyses the hydrolysis of GTP (guanosine triphosphate) to initiate a cell signal. The hydrolysis is facilitated by GTPase Activating Protein (GAP) and Guanine Nucleotide Exchange Factor (GEF) with subsequent conversion to inactive K-ras GDP (Guanosine diphosphate) complex. On the left is represented the k-ras Wild type (WT) environment which prevents the translation of viral transcripts and abrogates reovirus assembly. The double stranded RNA (dsRNA) dependant protein kinase R (PKR) plays a crucial role in the viral translation block by dimerization and autophosphorylation to Phospho-PKR (P-PKR) which in turn promotes the phosphorylation of translation initiation factor 2 subunit Alpha (eIF2α) to P-eIF2α. The dashed arrow depicts the translational inhibition by P-eIF2α in K-ras WT cells. In K-ras compromised situation (shown on right) the autophosphorylation of P-PKR is inhibited with unabated viral translation and subsequent virion assembly and cell lysis. The factors contributing to the cross talk between defective K-ras and compromised PKR phosphorylation are unknown and thus represented by (?). Cancer up-regulatory gene 2 (CUG2) frequently elevated in variety of tumor tissues have been recently characterized to promote reoviral proliferation by activation of K-ras and inhibition of PKR phosphorylation. CUG2 has been proposed as a biomarker to identify the subset of cancers that might benefit from reoviral therapy.
The recently discovered novel oncogene CUG2 (cancer upregulatory gene-2), inhibits the expression of PKR and activates Ras and p38 MAPK(19). Studies further confirmed that inhibition of p38 MAPK or Ras blocks reoviral proliferation even in the presence of CUG2 indicating the possibility of multimodal cross talk between Ras activation and inhibition of PKR phosphorylation (19). It is pertinent to mention that PKR dimerization and autophosphorylation is critical for effective viral propagation but the definitive contribution of Kras mutation in the process is still elusive. Aberrant cell signaling cascade generates many anomalous events within the cell and exactly how it facilitates the inhibition of PKR dimerization is yet to be defined clearly.
Investigation of the mechanism of apoptosis in reovirus infected HEK293 cell line concluded a significant role of TRAIL (TNF-related apoptosis-inducing ligand). The apoptotic event was successfully inhibited by anti-TRAIL antibodies or death receptor (DR)4 and DR5 (20). Similar involvement of TRAIL was confirmed in cell lines derived from two different human lung (A157/ H549) and breast cancers (MDA231/ ZR75-1) (20, 21). Reovirus infection synergistically sensitizes these cancer cell lines to killing by exogenous TRAIL. The observed sensitization was associated with an increase in the activity of the death receptor-associated initiator caspase 8, and was inhibited by the peptide IETD-fmk, suggesting that reovirus sensitizes cancer cells to TRAIL-induced apoptosis in a caspase 8-dependent manner. Enhanced sensitization was also found to be associated with increased cleavage of PARP, a substrate of the effector caspases 3 and 7 (22).
In vivo experience using animal models of cancer
Tumor regression studies in animals with reovirus as single agent showed dramatic results. Pronounced effects often with complete tumor regression was documented in vivo in two subcutaneous (P=0.0002 for both U251N and U87) and in two intracerebral (p=0.0004 for U251N and P=0.0009 for U87) human malignant glioma mouse models (4). Immuno-competent C3 mice implanted with Ras-transformed C3H10t1/2 fibroblast showed complete regression of tumor after ITu injection of reovirus (23). Similar strategy of ITu injection resulted in significant tumor regression in SCID mice with subcutaneously implanted v-erbB transformed NIH-3T3 cells (23), and in SCID-NOD mice with subcutaneously implanted human malignant glioma (4, 8). Subcutaneous tumor allograft studies in immune competent C3H mice showed strong inhibition of tumor growth with intravenous administration of reovirus (15). A 74% cure rate was observed in comparatively less immune-compromised nude mice harboring human malignant glioma with single intra lesion injection of reovirus. SCID-NOD mice bearing subcutaneous U251N xenografts when treated with single injection of live or dead reovirus demonstrated a striking regression of live-virus-treated tumors (24). Immunofluorescence analysis showed that reovirus replication was restricted to the tumor mass without spreading to the underlying normal tissue.
Reovirus as combination therapy
Association with Radiotherapy
Combination therapy involving radiation and reovirus was evaluated both in vitro and in vivo (21). The CRC cell line HCT 116 was treated with reovirus and radiation individually, and in combination, demonstrated a marked synergy in the combinational subset. In vivo murine studies depicted similar synergy in nude mice with tumor induced by HCT116/SW480 cell implantation as well as in C57BL6 mice by B16 melanoma cells mediated tumor induction (21).
The relative tumor volume (RTV) when measured in nude mice xenografted with RH30 and SK-ESI human sarcoma and treated with similar doses revealed lowest mean tumor volume and longest event free survival in the group receiving the combination treatment. TUNEL assay performed with tumor biopsies showed enhanced apoptotic activity in combination therapy as compared to single agent (25).
Association with Chemotherapy
In vitro studies to determine augmentation of viral therapy with chemotherapy was done using gemcitabine, fluorouracil, cisplatin, and doxorubicin in HCT116 cells. Reovirus was synergistic with all four drugs across a wide range of concentration (26). The synergistic consequences observed with gemcitabine advanced further onto in vivo evaluation of xenografted nude mice. A remarkable synergy was confirmed with no residual tumor tissues remaining at the end point of the study in more than 95% of the mice (27).
Another independent study of reovirus in combination with cisplatin, mytomycin, vinblastine, or gemcitabine in NSCLC cells lines documented synergy in cell lines sensitive to the chemotherapeutic drugs and antagonism in cell lines that are drug resistant. The taxanes showed reasonable synergy when administered in combination even in drug resistant cell lines (28). Similarly C57BL/6 rodent with B16 melanoma induced tumor when treated by combination therapy of reovirus and cisplatin showed a synergistic reduction in RTV as compared to those receiving monotherapy (29).
In context of animal models of human tumors, athymic mice bearing xenografts of osteosarcoma, Ewings and synovial sarcoma when treated in similar fashion also confirmed a synergistic antitumor activity in comparison to monotherapy (30). Docetaxel administered in combination in cell lines and PC-3 prostate cancer mouse model confirmed synergy which diminished with increasing docetaxel concentration (31). The confinement of viral proteins within the tumor mass was confirmed by several studies including immunohistochemical evaluation of U87xenografted rodent (8). The broad oncolytic spectrum of reovirus as single agent and in combination along with remarkable success of the animal study platform provided the scientific confidence to proceed onto clinical trials. Although much preclinical work has been carried out establishing that reovirus could serve as a cancer therapy, the mechanisms governing the permissiveness of transformed cells to reovirus infection remain to be fully characterized. Furthermore, as reovirus replicates in cancers of very diverse origin, it is likely that the virus exploits cellular signals that occur often in transformation and tumorigenesis. A deeper understanding of the molecules involved in anti-viral defense and the patterns they recognize will allow harnessing them for better therapeutic strategies. Thus, delineating this usurpation should in turn shed light on signaling that is common among various cancers, and may reveal novel therapeutic targets.
Although the first hint of Reovirus susceptibility (32) was noted in 1977, it was not until 1990s that any scientific clue was obtained regarding the preferential replication of Reovirus in transformed cells. Since then systematic research has provided some clues on to the molecular synergy of efficient reovirus oncolysis in ras compromised cancer cells. Although the preliminary finding might not be substantial in defining the viral tropism, it has nevertheless logically permitted clinicians to attempt the use of reovirus as treatment towards ras mutated carcinogenicity especially in situation where no other well established treatment regime is available.
Incidentally, reovirus is intrinsically oncolytic without need for any genetic manipulation. It is a naturally occurring virus that possesses the right level of potency that renders it relatively harmless to normal cells while having strong lytic effect on ras-compromised cancer cells. Investigations on the mechanisms of selective viral oncolysis in ras- mutated tumor cells will help unravel the complex cellular pathways involved in cellular transformation which in turn will not only help in identifying novel targets for cancer therapy but also shed light on which cancer backgrounds are compatible with viral oncolysis strategies.
CLINICAL EXPERIENCE WITH REOVIRUS
Reovirus has been therapeutically tested in 332 (as reported) patients administered ITu or intravenously (IV) either as a monotherapy or in combination with radiotherapy or chemotherapy. The first human trial with reovirus began in July 2001, and, a total of 27 clinical trials (phase I/II/III) have been completed/initiated/planned as Phase 1, 2 and 3. Thirteen trials have been completed, while 12 are ongoing, and 2 have been announced. Of these 27 trials, 6 will be sponsored by the National Cancer institute (NCI). Of the 17 trials with reported clinical data, clinically formulated reovirus was injected ITu in 5 trials enrolling 75 patients, while the remaining 12 clinical trials involved the intravenous route enrolling 257 patients (table 1). The current dosing regimen is an IV of 3×1010 TCID50 on days 1–5 over 60 minutes repeated every 3 or 4 weeks in both mono- and combination therapy. As with the preclinical observations, the clinical experience with reovirus has been interesting, evolving and has shown promise. Summaries of the results are provided in table 1.
Table 1. Summary of Clinical Trials with reported data.
This table summarizes the current data from completed and ongoing clinical trials with Reovirus. The study # preceded by “REO” indicates the number assigned by the study sponsor, namely Oncolytics, to enable an easy search for the reader. While some trials are mature and full reports have been published, others are ongoing with preliminary data presented at international and national meetings or updated on the company’s website. The table provides a quick overview with focus on the study design, patient accrual numbers, dosing, immunology, toxicities, and efficacy, and comments about the uniqueness of the conduct or the results of the study.
| Study # | Indication | Other therapy | Number Patients |
Phase | Lowest Dose (TCID50) |
Highest Dose (TCID50) |
Grade 3/4 toxicities | Efficacy | Viral Detection Serum/CSF/Stool/ Urine/Feces |
Antibody Response |
Reference | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intra Tumoral | ||||||||||||
| 1 | Solid tumors | 18 | 1 | 10*7 (PFU) | 10*10 (PFU) | None | 1 CR, 1 PR, 8 SD | Serum | Yes | 33 | ||
| 2 | Prostate | 6 | Pilot | 10*7 (PFU) | None | 1 PR, 4 SD | Urine | Yes | 34 | |||
| 3 | Malignant Gliomas | 12 | 1 | 1 × 10*7 | 1 × 10*9 | Transient GGT elevation | 1 SD | Saliva, Feces | Yes | |||
| 6 | Solid tumors | XRT 20–36 Gy | 23 | 1 | 1 × 10*8 | 1 × 10*10 | None | 7 PR, 7 SD | None | Yes | 39 | |
| 7 | Malignant Gliomas | 15 | 1//2 | 1 × 10*8 | 1 × 10*10 | NR | NR | NR | NR | |||
| 8 | Solid tumors | XRT-20 Gy | 16 | 2 | 1 × 10*10 | None | 4 PR, 2 MR, 7 SD | NR | Yes | 40 | ||
| Intravenous | ||||||||||||
| 4 | Solid tumors | 18 | 1 | 1 × 10*8 | 3 × 10*10 | None | 1 PR, 7 SD | Urine, Serum, Saliva, Feces | Yes | 41 | ||
| 5 | Solid tumors | 33 | 1 | 1 × 10*8 | 3 × 10*10 | Flu like syndrome CPK-MB/Trop I elevation Lymphopenia Neutropenia | 3 MR, 7 SD | Urine, Serum, Saliva, Feces | Yes | 44 | ||
| 9 | Solid tumors | Gemcitabine 1000 mg/m2 | 12 | 1 | 1 × 10*9 | 3 × 10*10 | Flu like syndrome Neutropenia AST/ALT/GGT elevation Trop I elevaton/ST changes | 48 | ||||
| 10 | Solid tumors | Docetaxel 75 mg/m2 | 24 | 1 | 1 × 10*9 | 3 × 10*10 | Febrile neutropenia Thrombocytopenia GI symptoms Hypokalemia Hypotension | 4 PR, 3 MR, 7 SD | Urine, Serum, Saliva, Feces | Yes | 54 | |
| NR | NR | |||||||||||
| 11 | Solid tumors | Carboplatin AUC 5 Paclitaxel 175 mg/m2 | 31 | 1//2 | 3 × 10*9 | 3 × 10*10 | Myelosuppression Hypotension | 8 PR, 6 SD | 56 | Efficacy data among 19 patients with Head and Neck Cancer | ||
| 13 | Colorectal Cancer with liver metastases | 10 | 2 | 1 × 10*10 | Yes | 16 | Virus selectively detected in cancerous liver tissue | |||||
| 14 | Sarcomas | 53 | 2 | 3 × 10*10 | Neutropenia Optic neuritis | 19 SD | NR | NR | ||||
| 15 | Head and Neck Cancer | Carboplatin AUC 5 Paclitaxel 175 mg/m2 | 14 | 2 | 3 × 10*10 | Neutropenia Hypokalemia Fatigue Anemia Nausea Hypotension | 4 PR, 2 SD | NR | NR | 57 | ||
| 16 | NSCLC | Carboplatin AUC 6 Paclitaxel 200 mg/m2 | 22 | 2 | 3 × 10*10 | Myelosuppression Hypotension Fatigue GI symptoms | 6 PR, 13 SD | NR | NR | 58 | ||
| 17 | Pancreatic Cancer | Gemcitabine 800 mg/m2 | 18 | 2 | 3 × 10*10 | Neutropenia | 1 unconfirmed PR 7 SD | NR | NR | 52 |
Abbreviations: AUC: area under the concentration time curve; XRT: radiation; PR: partial response; MR: minimal response; SD: stable disease; NR: not reported; NSCLC: non small cell lung cancer; PFU: particle forming units; TCID: tissue culture infective dose
Reovirus as intratumoral injections
The first human phase 1 trial was conducted in 18 patients with metastatic or recurrent solid tumors with easy access to the tumor to allow for direct ITu injection, and measurement by direct observation or palpation. The treatment was well tolerated without any observation of dose limiting toxicity (DLT) and/or maximum tolerated dose (MTD) (33). The second trial, was based on the preclinical experience of reovirus on prostate derived cell lines (34). Six prostate cancer patients (stage T2/T3) received a single trans-rectal ultra-sonography (TRUS) guided reovirus injection followed by radical prostatectomy after 3 weeks. Histological analysis after prostatectomy of the injected lesions and other synchronous lesions demonstrated CD8 T-cell infiltration and evidence of caspase 3 activity within the reovirus injected areas. The third clinical trial was based on the preclinical data showing reovirus activity in malignant gliomas (4). While oncolytic viruses have been used in clinical trials in malignant gliomas (35–38), this study was the first to utilize reovirus. A total of 12 patients (10 glioblastoma multiforme, 1 anaplastic astrocytoma and 1 anaplastic oligoastrocytoma) were treated at 3 dose-escalating levels (ongoing trial)‥ Antibody studies showed a seroconversion rate of 83% consistent with other clinical studies. Subsequently, a combined phase 1/2 study (currently ongoing) was started in recurrent malignant gliomas with a single ITu infusion (utilizing transcranial catheters) of reovirus directly into the intracerebral tumor over 72 hours. Accrual to the phase I portion has been completed, but the data are pending.
In another two-stage phase I trial, dose escalating reovirus was given ITu along with palliative radiotherapy to 23 patients with advanced cancers (39). There was no evidence of exacerbation of skin toxicity induced by radiation. There was marked efficacy with 7 of 14 evaluable patients having experienced a partial response (PR). In another phase 2, multicenter, clinical trial, ITu reovirus was evaluated when combined with low-dose radiotherapy (20 Gy) in advanced cancer patients (40). This study demonstrated safety and efficacy of the combination with 4 patients with PR and 2 with minimal response.
Reovirus as systemic administration
There have been two single agent trials of reovirus as a systemic IV infusion. One clinical trial was conducted at our center, Montefiore Medical Center, Albert Einstein College of Medicine (41). This was a single center dose escalation trial and enrolled 18 patients with refractory solid tumors. Overall, toxicities were minor, with only 2 patients experiencing grade 2 events, without an observation of a single DLT. One patient with breast cancer who received 7 cycles, developed grade 2 fever and grade 1 chills that progressively worsened with repeated administration. She had a PR and her tumor had a mutation in ras codon 12 (42, 43). In the second systemic phase I study of reovirus as single agent, the 33 patients entered, of which 10 patients showed stable disease (SD) (44). Overall, 28 patients showed an increase in neutralizing anti-reovirus antibody (NARA) titers to a maximum at 4 weeks, which remained constant during subsequent cycles (45). In spite of the NARA response, which could have blunted the viral delivery to tumor site (via rapid neutralization), viable virus was demonstrated in post treatment biopsies after 2 cycles (45).
More recently, in a study that enrolled patients with advanced CRC patients with liver metastasis, reovirus was given IV for 5 consecutive weeks before the planned liver metastesectomy. Among the 10 patients treated so far, there is evidence that reovirus selectively targets tumor cells versus normal liver cells (60% patients have shown no evidence of reovirus in their normal liver cells). In 2 patients only necrotic tissue was seen while reovirus was detected in the immune cells of the tumor of 1 patient. This new exciting information demonstrates that reovirus can be delivered specifically and selectively as a monotherapy while sparing the normal liver cells (ongoing trial)‥ The first completed phase II trial of single agent reovirus targeted 53 patients with soft tissue and bone sarcomas with metastasis to lung (ongoing trial). A total of 19 patients showed SD. One patient with synovial sarcoma had SD for over 80 weeks.
There is strong preclinical rationale to combine intravenous infusion of reovirus with cytotoxic chemotherapy like gemcitabine, carboplatin or docetaxel (15, 27, 28, 46, 47). The phase 2 dose of reovirus was based on two-phase 1 monotherapy trials (41, 44). It was postulated that chemotherapy might blunt the immune mediated viral clearance without significantly increasing the toxicity profile. In the first combination phase I study, intravenous reovirus (on day 1–5 of each cycle with the starting dose at 3 × 109 TCID50) was administered with gemcitabine at 1000 mg/m2 on day 1 and day 8 (21 day cycle) in dose escalating cohorts (48). However, with the observation of 2 DLTs [grade 3 ALT (alanine aminotransferase) rise and grade Troponin I] the dose of reovirus was amended to a 1 day treatment. One patient with nasopharyngeal carcinoma [(NPC) which is caused by an Epstein Barr virus (EBV) infection] demonstrated a PR, possibly due to the expression of EGFR (EBV induced expression). The combination showed clinical activity in spite of the potential NARA response, and ongoing work is unraveling the intricate details of mechanisms involved in reovirus medicated oncolysis and resistance (49, 50). Based on this clinical (48) and prior preclinical findings (51), a phase 2 clinical trial in patients with metastatic pancreatic cancer is ongoing and preliminary data suggests a clinical benefit rate of 58% with prolonged SD (52). Preclinical data suggestive of hepatic toxicity associated with reovirus (53) and the observation of the ALT elevation when combined with gemcitabine (48) should be factored in when dealing with situations where patients have compromised hepatic function or are taking potentially hepatotoxic medications, such as acetaminophen.
Therapeutically formulated reovirus has been combined with docetaxel in 24 patients with advanced cancer (54). Of all patients treated, 46% experienced grade 3 or greater neutropenia, consistent with that observed with docetaxel monotherapy (65%) at the same dose and schedule. MTD was not technically reached as only one DLT, of grade 4 neutropenia, was encountered. Four PR were observed in breast, stomach, gastroesophageal, and ocular melanoma along with three minor responses in mesothelioma, prostate cancer, and squamous cell cancer of the head and neck. Only two patients showed evidence of viral shedding. Pharmacokinetic studies revealed no change in docetaxel clearance by the addition of reovirus. Docetaxel showed no effect on NARA, a finding which was inconsistent with preclinical data (55).
In a separate phase I trial, reovirus was added to the combination of carboplatin and paclitaxel (56). With preliminary signs of clinical activity, the phase 2 expansion cohort was enriched with patients with squamous cell carcinomas of head and neck (SCCHN), and remarkably, 8 of 19 (42%) evaluable patients showed PR. The toxicities were consistent with earlier results. This study demonstrated the safety and efficacy of this combination, leading to a targeted phase II study in patients with SCCHN with a RR of 31% (57), and finally to a randomized phase III study of carboplatin and paclitaxel with or without Reovirus in patients with platinum refractory SCCHN (ongoing trial). The same combination has also been tested in patients with advanced non small cell lung cancer (58). As reported at the 14th World Lung Cancer Congress, the combination was well tolerated and of the 23 patients entered, 6 patients had a PR, and 13 had SD.
Neutralizing Anti-Reovirus Antibodies (NARA)
An important and critical issue when considering the clinical use of reovirus is the presence of pre-existing, and the development of a rapid massive rise of titer of “on therapy” neutralizing anti-reovirus antibodies (NARA). This phenomenon of NARA development has been consistently observed across the single agent and in the chemotherapy combination trials. It is clearly evident that in spite of the presence of pre-existing NA, there is a detectable 100s of fold increase in the viral titer, which has been suggested to be both desirable and unwarranted. On the one hand, it allows for limiting the toxic effects of the virus and protects patients from the unwanted side effects of the virus infection, while on the other it may compromise its beneficial potent anti-cancer effects. Prior pre clinical in vivo studies in murine models using C3H mice have shown that blunting the immune response using cyclosporine A or anti CD4/CD8 antibodies leads to improved efficacy (15). Similarly, cyclophosphamide when used as a modulating agent, was effective in ensuring tumor seeding of the IV delivered reovirus in tumors, an entity which was previously not attainable (59). By modifying the dose of the cyclophosphamide, the authors also demonstrated that it modulates but does not ablate the NARA response.
The concomitant delivery of reovirus with chemotherapy has been purported to be of benefit partly by attenuation of the NARA response. However, in combination chemotherapy trials, specifically with docetaxel, and carboplatin-paclitaxel, there was a significant rise on NA titer and no discernable increase in toxicity (46, 56). Another interesting phenomenon observed in these trials was that in comparison to single agent studies, the rate of rise of the NA titer was lower, thereby leading to a delay in the achievement of the peak titer. Slower development of NA may have beneficial effects by enhancing tumor seeding of the virus leading to greater efficacy, without compromising safety. Interestingly, the only agent that had some suggestion of the ability to blunt the NA response was gemcitabine (48). This is not surprising since gemcitabine has been shown in murine models to specifically affect the generation of antibodies by B cells (49) and to suppress myeloid suppressor cells (60). Furthermore, independent of the attenuation of the NARA response, gemcitabine has been reported to tip cellular immunity favoring reovirus initiated anti tumor immune response (51, 60). The attenuated NARA response allowed for greater virus replication leading to greater toxicity of the combination as compared to the single agent profile, an important but not entirely unexpected clinical observation. Therefore, careful monitoring of immune function should remain an important consideration in future combination trials. Moreover, it has been suggested that because of the interference of the adaptive immunity of the host, the most effective systemic delivery of reovirus, will be achieved through rapid, repeated high doses of virus within the first week of treatment, before the NARA response has been boosted (45). The current dosing schema of 5 continuous daily IV injections of the virus every 3–4 weeks, therefore has some clinical and scientific rationale.
SUMMARY, CONCLUSIONS, AND THE FUTURE
In summary, reovirus has shown promise with far reaching implications for future drug development. While initially developed as an anticancer agent based on the scientific premise that viral replication is supported in ras driven cancer cells; the paradox lies in that the clinical development has not been driven by this fact. Moreover, clinical benefit has also been observed in patients with tumors where the incidence of ras mutations has historically been very low. This clearly suggests that a second important clinical phenomenon is underway in promoting virus efficacy. As discussed extensively earlier in this review, evidence suggests that reovirus, similar to other viruses, manipulates the immune system to mount an anti cancer response. As intriguing as this fact is, it only further affirms that the translation of science from “bench” to “bedside” is challenging, to say the least.
Further characterization of the activity of the virus is clearly required and it is imperative that it be in the form of a concerted effort of laboratory scientists with an interest in tumor biology, virologists, physician scientists, and academic clinicians, with a common sense of purpose. It is also critical that the patient be entirely integrated into this effort. The availability of tumor tissue to study both, the effect of this therapy, and the biology behind the driving force of the cancer is absolutely essential and cannot be overstated.
It is important to note here that using viruses to target cancer is not a new phenomenon. In fact, viral approaches to cancer have been attempted for over half a century with little success (61, 62). One of the most extensively studied products is ONYX-015, an attenuated E1B-55K chimeric human group C adenovirus, which preferentially replicates within and lyses tumor cells that are p53 negative (12). Almost a decade later another novel adenovirus mutant, ONYX-053, was created that demonstrated that loss of E1B-55K-mediated late viral RNA export, rather than p53 degradation, restricts ONYX-015 replication in primary cells. It was experimentally proved that in contrast to the initial hypothesis, tumor cells that support ONYX-015 replication provide the RNA export function of E1B-55K.(63). There was great enthusiasm for this approach when a similar product – the genetically modified adenovirus H101, made by Shanghai Sunway Biotech obtained commercial approval in China in the treatment of advanced head and neck cancer (64). Once again, despite the promises of early in vivo lab work suggesting tumor specificity, these viruses do not specifically infect cancer cells; however they still retain some preferential cell kill for cancer cells. As of the last report, response rates were approximately doubled for H101 plus chemotherapy as compared to chemotherapy alone; however survival information is unknown. Another limitation of this approach is that it appears to render maximum benefit when given as a direct ITu injection and the patient experiences a febrile response (65). Another virus in late stage clinical development is Oncovex, a second generation oncolytic herpes simplex virus that expresses GM-CSF (granulocyte macrophage - colony stimulating factor) and in which deletion of ICP 34.5 provides tumur selectivity. This has been tested as a single agent ITu therapy and in combination with radiotherapy (66, 67). It is currently in phase III clinical development to test efficacy in malignant melanoma (ongoing trial).
Other approaches using virus mediated oncolysis have included the use of the oncolytic adenovirus ICOVIR-5 as a treatment for malignant gliomas, PV 701, an attenuated form of the Newcastle virus (68, 69) and the vesicular stomatitis virus (70–73). Even more intriguing, the measles virus is also being evaluated as an oncolytic virus, with encouraging data in breast and ovarian cancer (74–76). The reader is referred to more detailed reviews on oncolytic viruses (77–79). These examples of viral oncolytic therapy elucidate the multiple challenges that we face in developing new viral therapies for cancer, including reovirus.
Reolysin® is the trade name of the therapeutic version of human reovirus formulated and developed by Oncolytics Biotech Inc. (Alberta, Canada). It is a translucent light blue liquid containing a purified isolate of 1×1011 TCID50 (tissue culture infective dose) of replication competent reovirus serotype 3 Dearing strain per milliliter in a phosphate buffered solution and is used in many of the clinical trials. The virus in its clinical formulation as Reolysin has rapidly progressed through clinical development to a phase III trial in platinum refractory SCCHN (ongoing trial) An interesting pathway of the clinical development of reovirus lies in metastatic CRC; where patients whose tumors harbor a mutation in the Kras oncogene are ineligible to receive the anti EGFR monoclonal antibodies, cetuximab and panitumumab (80, 81). Based on preclinical in vivo data that the combination of reovirus and irinotecan is particularly synergistic in the ras mutant cancer cells (82), the phase I study of the combination of FOLFIRI (FOLinic acid, 5-Fluoro-Uracil, and IRInotecan) with reovirus is currently targeting patients with a Kras mutation, with the potential to fulfill an unmet medical need. As mentioned earlier preclinical observations of preferential viral tropism under specific mutational staus is not always replicable in human subjects. The targeting of reoviral therapy on Kras mutated mCRC subjects is not selected on the basis of the preclinical promises of enhanced virulence under Kras conditions but rather due to lack of any FDA approved therapy for platinum refractory mCRC subset of patients.
This clearly exemplifies the future of drug development: the potential of a safe and effective drug whose development is biomarker driven. The current climate of research and health care in the US is at a crossroads with major changes expected in the manner that health care is delivered by caregivers, received by patients, and paid for by third party payers, including the governments and the private insurance plans. The success or failure of a drug being tested in clinic will be highly dependent on its absolute effectiveness in an appropriate patient profile based on a validated biomarker. This is clearly a “win win” situation for all the parties involved: the patient only receives the medication that is highly likely to benefit him/her, thereby also avoiding unnecessary toxicities, and will also bring down the cost of healthcare by paying only for effective therapies. A word of caution is appropriate here: in spite of the encouraging development of reovirus so far, the “proof of the pudding is in the eating”, and unless it can demonstrate improvement and patient benefit over the current standard of care in a well conducted phase III trial, it will be relegated to the confines of history, as have many of its predecessors.
REFERENCES
- 1.Sabin AB. Reoviruses. A new group of respiratory and enteric viruses formerly classified as ECHO type 10 is described. Science. 1959;130:1387–1389. doi: 10.1126/science.130.3386.1387. [DOI] [PubMed] [Google Scholar]
- 2.Nelson NJ. Viruses and cancer. J Natl Cancer Inst. 1999;91:1709. doi: 10.1093/jnci/91.20.1709. [DOI] [PubMed] [Google Scholar]
- 3.Min HJ, Koh SS, Cho IR, et al. Inhibition of GSK-3beta enhances reovirus-induced apoptosis in colon cancer cells. Int J Oncol. 2009;35:617–624. [PubMed] [Google Scholar]
- 4.Wilcox ME, Yang W, Senger D, et al. Reovirus as an oncolytic agent against experimental human malignant gliomas. J Natl Cancer Inst. 2001;93:903–912. doi: 10.1093/jnci/93.12.903. [DOI] [PubMed] [Google Scholar]
- 5.Van Den Wollenberg DJ, Van Den Hengel SK, Dautzenberg IJ, Kranenburg O, Hoeben RC. Modification of mammalian reoviruses for use as oncolytic agents. Expert Opin Biol Ther. 2009;9:1509–1520. doi: 10.1517/14712590903307370. [DOI] [PubMed] [Google Scholar]
- 6.Norman KL, Coffey MC, Hirasawa K, et al. Reovirus oncolysis of human breast cancer. Hum Gene Ther. 2002;13:641–652. doi: 10.1089/10430340252837233. [DOI] [PubMed] [Google Scholar]
- 7.Shmulevitz M, Marcato P, Lee PW. Unshackling the links between reovirus oncolysis, Ras signaling, translational control and cancer. Oncogene. 2005;24:7720–7728. doi: 10.1038/sj.onc.1209041. [DOI] [PubMed] [Google Scholar]
- 8.Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 9.Mundschau LJ, Faller DV. Endogenous inhibitors of the dsRNA-dependent eIF-2 alpha protein kinase PKR in normal and ras-transformed cells. Biochimie. 1994;76:792–800. doi: 10.1016/0300-9084(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 10.Hata Y, Etoh T, Inomata M, et al. Efficacy of oncolytic reovirus against human breast cancer cells. Oncol Rep. 2008;19:1395–1398. [PubMed] [Google Scholar]
- 11.Errington F, White CL, Twigger KR, et al. Inflammatory tumour cell killing by oncolytic reovirus for the treatment of melanoma. Gene Ther. 2008;15:1257–1270. doi: 10.1038/gt.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 13.Errington F, Steele L, Prestwich R, et al. Reovirus activates human dendritic cells to promote innate antitumor immunity. J Immunol. 2008;180:6018–6026. doi: 10.4049/jimmunol.180.9.6018. [DOI] [PubMed] [Google Scholar]
- 14.Zitvogel L. Dendritic and natural killer cells cooperate in the control/switch of innate immunity. J Exp Med. 2002;195:F9–F14. doi: 10.1084/jem.20012040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirasawa K, Nishikawa SG, Norman KL, et al. Systemic reovirus therapy of metastatic cancer in immune-competent mice. Cancer Res. 2003;63:348–353. [PubMed] [Google Scholar]
- 16.Adair RA, Roulstone V, Scott KJ, et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci Transl Med. 2012;4:138ra77. doi: 10.1126/scitranslmed.3003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. Embo J. 1998;17:3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bischoff JR, Samuel CE. Mechanism of interferon action. Activation of the human P1/eIF-2 alpha protein kinase by individual reovirus s-class mRNAs: s1 mRNA is a potent activator relative to s4 mRNA. Virology. 1989;172:106–115. doi: 10.1016/0042-6822(89)90112-8. [DOI] [PubMed] [Google Scholar]
- 19.Park EH, Park EH, Cho IR, et al. CUG2, a novel oncogene confers reoviral replication through Ras and p38 signaling pathway. Cancer Gene Ther. 2010;17:307–314. doi: 10.1038/cgt.2009.83. [DOI] [PubMed] [Google Scholar]
- 20.Clarke P, Meintzer SM, Gibson S, et al. Reovirus-induced apoptosis is mediated by TRAIL. J Virol. 2000;74:8135–8139. doi: 10.1128/jvi.74.17.8135-8139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Twigger K, Vidal L, White CL, et al. Enhanced in vitro and in vivo cytotoxicity of combined reovirus and radiotherapy. Clin Cancer Res. 2008;14:912–923. doi: 10.1158/1078-0432.CCR-07-1400. [DOI] [PubMed] [Google Scholar]
- 22.Barkett M, Gilmore TD. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- 23.Strong JE, Lee PW. The v-erbB oncogene confers enhanced cellular susceptibility to reovirus infection. J Virol. 1996;70:612–616. doi: 10.1128/jvi.70.1.612-616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Qian H, Yu J, et al. Administration of PUMA adenovirus increases the sensitivity of esophageal cancer cells to anticancer drugs. Cancer Biol Ther. 2006;5:380–385. doi: 10.4161/cbt.5.4.2477. [DOI] [PubMed] [Google Scholar]
- 25.Gidwani P, Zhang W, Liu L, et al. Radiation in combination with Reolysin for pediatric sarcoma[Abstract]. Proceedings of the 99th Annual Meeting of the American Association for Cancer Research; 2008: April 12–16; San Diego (CA). Poster #3751. [Google Scholar]
- 26.Wadler S. The oncolytic reovirus, reolysin augments the anticancer effects of cytotoxic agents in vitro against the ras-mutated human colon cancer cell line HCT116. Annual Meeting of the American Association for Cancer Research; 2004. [Google Scholar]
- 27.Lane ME. In vivo synergy between oncolytic reovirus and gemcitabine in ras-mutated human HCT-116 xenografts. Annual Meeting of the American Association for Cancer Research; 2007; Poster # 4812. [Google Scholar]
- 28.Sei S, Mussio JK, Yang QE, et al. Synergistic antitumor activity of oncolytic reovirus and chemotherapeutic agents in non-small cell lung cancer cells. Mol Cancer. 2009;8:47. doi: 10.1186/1476-4598-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heineman Synesgistic antitumor activity of oncolytic reovirus and cisplatin in malignant melanoma. International Conference on Oncolytic viruses as cancer therapeutics; 2007; Arizona. Poster. [Google Scholar]
- 30.Gidwani Systemic administration of Reolysin inhibits growth of human sarcoma xenografts alone and in combination with cisplatin and radiation. Collective Tissue Oncology Society. 2008 Poster # 35039. [Google Scholar]
- 31.Heinemann L. Synergistic antitumor activity of oncolytic reovirus and docetaxel in a PC-# prostate cancer mouse model. European Organization for research and treatment of cancer, AACR. 2008 Poster#308. [Google Scholar]
- 32.Hashiro G, Loh PC, Yau JT. The preferential cytotoxicity of reovirus for certain transformed cell lines. Arch Virol. 1977;54:307–315. doi: 10.1007/BF01314776. [DOI] [PubMed] [Google Scholar]
- 33.Morris DG, Forsyth PA, Paterson AH, et al. A phase I clinical trial evaluating intralesional Reolysin (reovirus) in histologically confirmed malignancies. Proc Am Soc Clin Oncol. 2002;21 Calgary Canada. #abstr 92; [Google Scholar]
- 34.Thirukkumaran CM, Nodwell MJ, Hirasawa K, et al. Oncolytic viral therapy for prostate cancer: efficacy of reovirus as a biological therapeutic. Cancer Res. 2010;70:2435–2444. doi: 10.1158/0008-5472.CAN-09-2408. [DOI] [PubMed] [Google Scholar]
- 35.Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 36.Rampling R, Cruickshank G, Papanastassiou V, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 37.Papanastassiou V, Rampling R, Fraser M, et al. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9:398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- 38.Harrow S, Papanastassiou V, Harland J, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11:1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- 39.Harrington KJ, Karapanagiotou EM, Roulstone V, et al. Two-stage phase I dose-escalation study of intratumoral reovirus type 3 dearing and palliative radiotherapy in patients with advanced cancers. Clin Cancer Res. 2010;16:3067–3077. doi: 10.1158/1078-0432.CCR-10-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saunders M, Anthoney A, Coffey M, et al. Results of a phase II study to evaluate the biological effects of intratumoral (ITu) reolysin in combination with low dose radiotherapy (RT) in patients (Pts) with advanced cancers. J Clin Oncol. 2009;27(suppl) (abstr e14514) [Google Scholar]
- 41.Gollamudi R, Ghalib MH, Desai KK, et al. Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors. Invest New Drugs. 2010;28:641–649. doi: 10.1007/s10637-009-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 43.Ogino S, Kawasaki T, Brahmandam M, et al. Sensitive sequencing method for KRAS mutation detection by Pyrosequencing. J Mol Diagn. 2005;7:413–421. doi: 10.1016/S1525-1578(10)60571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vidal L, Pandha HS, Yap TA, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res. 2008;14:7127–7137. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- 45.White CL, Twigger KR, Vidal L, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther. 2008;15:911–920. doi: 10.1038/gt.2008.21. [DOI] [PubMed] [Google Scholar]
- 46.Heinemann L, Simpson GR, Boxall A, et al. Synergistic effects of oncolytic reovirus and docetaxel chemotherapy in prostate cancer. BMC Cancer. 2011;11:221. doi: 10.1186/1471-2407-11-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrington KJ, Vile RG, Melcher A, Chester J, Pandha HS. Clinical trials with oncolytic reovirus: moving beyond phase I into combinations with standard therapeutics. Cytokine Growth Factor Rev. 2010;21:91–98. doi: 10.1016/j.cytogfr.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lolkema MP, Arkenau HT, Harrington K, et al. A phase I study of the combination of intravenous reovirus type 3 Dearing and gemcitabine in patients with advanced cancer. Clin Cancer Res. 2011;17:581–588. doi: 10.1158/1078-0432.CCR-10-2159. [DOI] [PubMed] [Google Scholar]
- 49.Nowak AK, Robinson BW, Lake RA. Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemo-immunotherapy. Cancer Res. 2002;62:2353–2358. [PubMed] [Google Scholar]
- 50.Prestwich RJ, Errington F, Ilett EJ, et al. Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin Cancer Res. 2008;14:7358–7366. doi: 10.1158/1078-0432.CCR-08-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plate JM, Plate AE, Shott S, Bograd S, Harris JE. Effect of gemcitabine on immune cells in subjects with adenocarcinoma of the pancreas. Cancer Immunol Immunother. 2005;54:915–925. doi: 10.1007/s00262-004-0638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mita M, Wang Y, Sarantopoulos J, et al. A Study of REOLYSIN® in Combination with Gemcitabine in Patients with Advanced Pancreatic Adenocarcinoma. Molecular Targets and Cancer Theraputics. 2011 Nov 12–16;Volume 10(Issue 11) Supplement 1 Poster Board B55. [Google Scholar]
- 53.Maddox JF, Amuzie CJ, Li M, et al. Bacterial- and viral-induced inflammation increases sensitivity to acetaminophen hepatotoxicity. J Toxicol Environ Health A. 2010;73:58–73. doi: 10.1080/15287390903249057. [DOI] [PubMed] [Google Scholar]
- 54.Comins C, Spicer J, Protheroe A, et al. REO-10: a phase I study of intravenous reovirus and docetaxel in patients with advanced cancer. Clin Cancer Res. 2010;16:5564–5572. doi: 10.1158/1078-0432.CCR-10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14:3536–3544. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karapanagiotou EM, Roulstone V, Twigger K, et al. Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced malignancies. Clin Cancer Res. 2012;18:2080–2089. doi: 10.1158/1078-0432.CCR-11-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karnad AB, Haigentz M, Miley T, et al. A Phase II Study of Intravenous Wild-type Reovirus (REOLYSIN®) in Combination with Paclitaxel Plus Carboplatin in Patients with Platinum Refractory Metastatic and/or Recurrent Squamous Cell Carcinoma of the Head and Neck. Molecular Targets and Cancer Theraputics. 2011 Nov 12–16;Volume 10(Issue 11) Supplement 1 Poster Board C-22. [Google Scholar]
- 58.Villalona-Calero MA, Lam ET, Otterson GA, et al. Phase II study of reovirus with paclitaxel and carboplatin in patients with metastatic non-small cell lung cancer (NSCLC) who have Kras or EGFR-activated tumors. 14th World Lung Cancer Congress; 2011; (abstr MO15.08) [Google Scholar]
- 59.Qiao J, Wang H, Kottke T, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 61.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 62.Csatary LK. Viruses in the treatment of cancer. Lancet. 1971;2:825. doi: 10.1016/s0140-6736(71)92788-7. [DOI] [PubMed] [Google Scholar]
- 63.O'Shea CC, Johnson L, Bagus B, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 64.Garber K. China approves world's first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 65.Xia ZJ, Chang JH, Zhang L, et al. [Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus] Ai Zheng. 2004;23:1666–1670. [PubMed] [Google Scholar]
- 66.Coffin RS, Hu JC, Davis CJ, et al. Results of a Phase I/II clinical trial with OncoVEXGM-CSF, a second generation oncolytic herpes simplex virus. J Clin Oncol. 2005;23(No. 16S):3099. Part I of II (June 1 Supplement) [Google Scholar]
- 67.Harrington K, Hingorani M, Tanay M, et al. Phase I/II dose escalation study of OncoVexGM-CSF and chemoradiotherapy (CRT) in untreated stage III/IV squamous cell cancer of the head and neck (SCCHN) J Clin Oncol. 2009;27(suppl):15s. 2009 (abstr 6018) [Google Scholar]
- 68.Alonso MM, Gomez-Manzano C, Jiang H, et al. Combination of the oncolytic adenovirus ICOVIR-5 with chemotherapy provides enhanced anti-glioma effect in vivo. Cancer Gene Ther. 2007;14:756–761. doi: 10.1038/sj.cgt.7701067. [DOI] [PubMed] [Google Scholar]
- 69.Pecora AL, Rizvi N, Cohen GI, et al. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J Clin Oncol. 2002;20:2251–2266. doi: 10.1200/JCO.2002.08.042. [DOI] [PubMed] [Google Scholar]
- 70.Giedlin MA, Cook DN, Dubensky TW., Jr Vesicular stomatitis virus: an exciting new therapeutic oncolytic virus candidate for cancer or just another chapter from Field's Virology? Cancer Cell. 2003;4:241–243. doi: 10.1016/s1535-6108(03)00251-4. [DOI] [PubMed] [Google Scholar]
- 71.Stojdl DF, Lichty BD, tenOever BR, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 72.Stewart JH, 4th, Ahmed M, Northrup SA, Willingham M, Lyles DS. Vesicular stomatitis virus as a treatment for colorectal cancer. Cancer Gene Ther. 2011;18:837–849. doi: 10.1038/cgt.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bridle BW, Boudreau JE, Lichty BD, et al. Vesicular stomatitis virus as a novel cancer vaccine vector to prime antitumor immunity amenable to rapid boosting with adenovirus. Mol Ther. 2009;17:1814–1821. doi: 10.1038/mt.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hasegawa K, Nakamura T, Harvey M, et al. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin Cancer Res. 2006;12:6170–6178. doi: 10.1158/1078-0432.CCR-06-0992. [DOI] [PubMed] [Google Scholar]
- 75.Iankov ID, Msaouel P, Allen C, et al. Demonstration of anti-tumor activity of oncolytic measles virus strains in a malignant pleural effusion breast cancer model. Breast Cancer Res Treat. 2010;122:745–754. doi: 10.1007/s10549-009-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Russell SJ, Peng KW. Measles virus for cancer therapy. Curr Top Microbiol Immunol. 2009;330:213–241. doi: 10.1007/978-3-540-70617-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bell J. Oncolytic viruses: an approved product on the horizon? Mol Ther. 2010;18:233–234. doi: 10.1038/mt.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong HH, Lemoine NR, Wang Y. Oncolytic Viruses for Cancer Therapy: Overcoming the Obstacles. Viruses. 2010;2:78–106. doi: 10.3390/v2010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zeyaullah M, Patro M, Ahmad I, et al. Oncolytic Viruses in the Treatment of Cancer: A Review of Current Strategies. Pathol Oncol Res. 2012 doi: 10.1007/s12253-012-9548-2. [DOI] [PubMed] [Google Scholar]
- 80.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 81.De Roock W, Piessevaux H, De Schutter J, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508–515. doi: 10.1093/annonc/mdm496. [DOI] [PubMed] [Google Scholar]
- 82.Seetharam R, Coffey M, Klampfer L, Mariadason J, Goel S. The addition of Reolysin, an oncolytic reovirus, to irinotecan shows synergistic anti cancer activity in colorectal cancer cell lines. Proceedings of the 101st Annual Meeting of the American Association of Cancer Research; 2010, Mar 31 – April 3; Washington, DC, Philadelphia (PA). Abstract # 773. [Google Scholar]