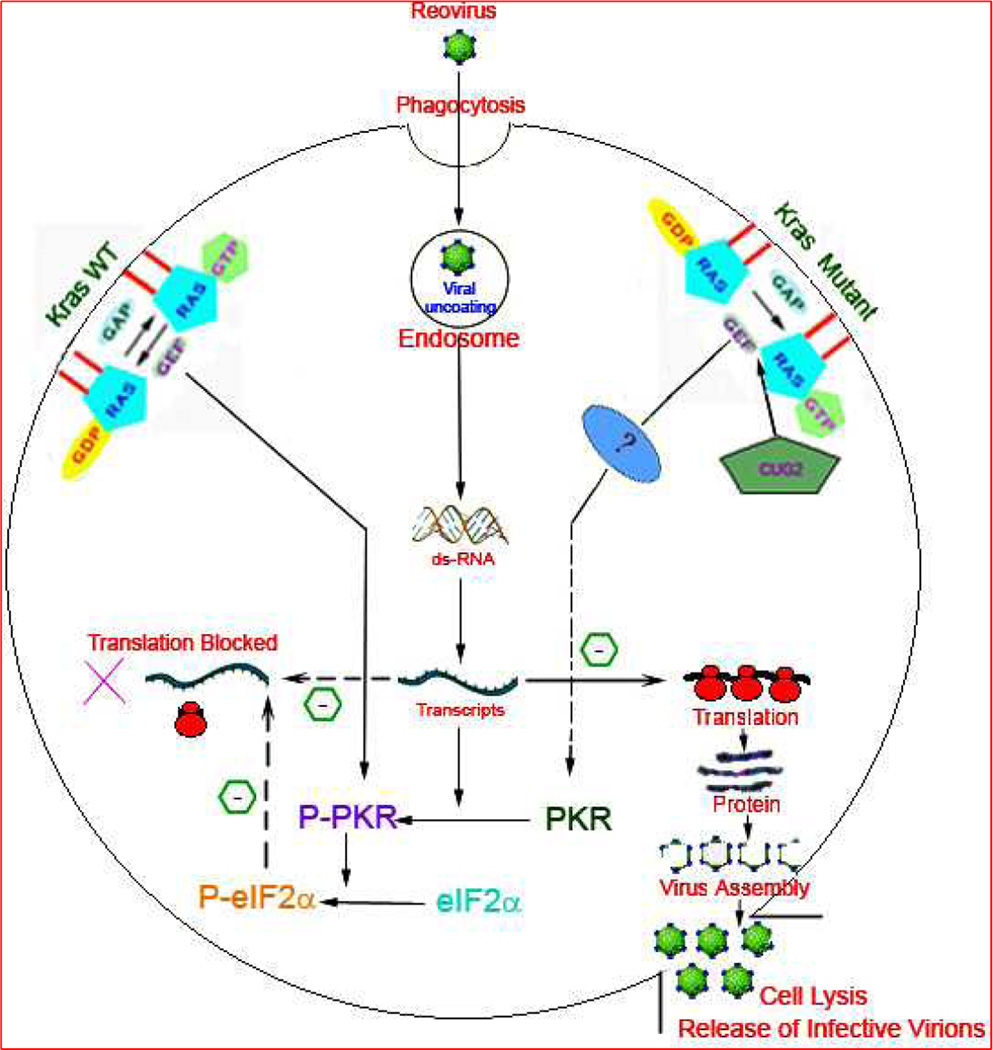
Figure 1. A schematic illustration of the current hypothesis of the fate of reovirus upon entering the host cell by phagocytosis.
The status of the host cell signaling cascade determines the downstream consequences. K-ras (Kirsten rat sarcoma viral Oncogene) is a membrane bound molecular switch which binds to and catalyses the hydrolysis of GTP (guanosine triphosphate) to initiate a cell signal. The hydrolysis is facilitated by GTPase Activating Protein (GAP) and Guanine Nucleotide Exchange Factor (GEF) with subsequent conversion to inactive K-ras GDP (Guanosine diphosphate) complex. On the left is represented the k-ras Wild type (WT) environment which prevents the translation of viral transcripts and abrogates reovirus assembly. The double stranded RNA (dsRNA) dependant protein kinase R (PKR) plays a crucial role in the viral translation block by dimerization and autophosphorylation to Phospho-PKR (P-PKR) which in turn promotes the phosphorylation of translation initiation factor 2 subunit Alpha (eIF2α) to P-eIF2α. The dashed arrow depicts the translational inhibition by P-eIF2α in K-ras WT cells. In K-ras compromised situation (shown on right) the autophosphorylation of P-PKR is inhibited with unabated viral translation and subsequent virion assembly and cell lysis. The factors contributing to the cross talk between defective K-ras and compromised PKR phosphorylation are unknown and thus represented by (?). Cancer up-regulatory gene 2 (CUG2) frequently elevated in variety of tumor tissues have been recently characterized to promote reoviral proliferation by activation of K-ras and inhibition of PKR phosphorylation. CUG2 has been proposed as a biomarker to identify the subset of cancers that might benefit from reoviral therapy.