Abstract
Given that mu opioid (MOP) and canabinoid (CB1) receptors are co-localized in various regions of the CNS and have been reported to associate as heteromer (MOP-CB1) in cultured cells has raised the possibility of functional, endogenous MOP-CB1 in nociception and other pharmacologic effects. As a first step in investigating this possibility, we have synthesized a series of bivalent ligands 1-5 that contain both mu agonist and CB1 antagonist pharmacophores for use as tools to study the functional interaction between MOP and CB1 receptors in vivo. Immunofluorescent studies on HEK293 cells co-expressing both receptors suggested 5 (20-atom spacer) to be the only member of the series that bridges the protomers of the heteromer. Antinociceptive testing in mice revealed 5 to be the most potent member of the series. As neither a mixture of monovalent ligands 9 + 10 nor bivalents 2-5 produced tolerance in mice, MOR-CB1 apparently is not an important target for reducing tolerance.
Introduction
The pharmacologic interaction between opioid and cannabinoid CB1 receptors has received considerable attention over the years. Both receptors are coupled to Gi/Go G-protein and they are expressed in overlapping areas of the CNS.1-5 Stimulation of either receptor with respective agonists inhibits adenylcyclase activity, blocks-voltage dependent calcium channels, activates potassium channels, and stimulates the MAP kinase cascade. Each receptor shares pharmacological properties that include analgesia, tolerance, and dependence upon chronic administration.6-8 Due to similar distribution of both receptors in pain processing areas, their interaction can play an important role in antinociception.9, 10, 11 For example, cross-tolerance between tetrahydrocanabinol and morphine, and the possibility that these receptors interact pharmacologically was demonstrated through antagonism of antinociception by naloxone or CB1 antagonist.12 Moreover, synergism between cannabinoids and opioids at sub-effective doses has been reported.13-18 Additionally, co-administration of morphine with a CB1 antagonist inhibited the development of both acute and chronic tolerance to morphine.19 Other evidence for interaction was obtained from self-administration studies showing that both receptors are involved in reward processes. In this regard, both CB1 antagonist 7 (SR 141716) 20 and opioid antagonist, naloxone, reduced self-administration of morphine or THC.21, 22 Furthermore, studies on CB1 knockout mice have shown that the dependence and reward properties were attenuated for morphine but not for other drugs of abuse.23 In mice lacking mu (MOP) and delta (DOP) opioid receptors, the cannabinoid withdrawal syndrome is reduced.24
Given that resonance energy transfer studies on co-expressing MOP and CB1 receptors in cultured cells have suggested they are organized as heteromer,25, 26 together with reports that these receptors are colocalized within neurons in rat nucleus accumbens27 and dorsal horn,28 raise the possibility that heteromeric MOP/CB1 receptors may be involved in some of the pharmacological interactions that have been reported.
As our laboratory has developed a number of selective ligands for investigating heteromeric GPCRs29-35, the present study was initiated to develop bivalent ligands as pharmacologic tools to study MOP-CB1 heteromer. The bivalent ligands designed for this purpose consist of a selective mu receptor agonist connected to a CB1 receptor-selective antagonist/inverse agonist via a spacer of varied length. Here we show in vitro evidence for bridging of protomers in a MOP-CB1 heteromer by a mu agonist/CB1 antagonist bivalent ligands and their pharmacologic properties in vivo.
Design Rational and Chemistry
The design rationale for targeting neighboring protomers in a MOP-CB1 heteromer was based on our studies with bivalent ligands that contain both agonist and antagonist pharmacophores for heteromeric opioid receptors.34-36 The MOP agonist pharmacophore is derived from α-oxymorphamine37
6 which was employed previously for several bivalent series. In light of reports38-39 that show a CB1 antagonist is capable of eliminating morphine tolerance, dependence, and reinstatement, the CB1 antagonist 7 was employed as a basis for the second pharmacophore in the bivalent ligand.20
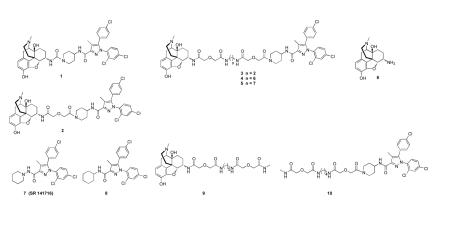
In prior studies we have explored the relationship between spacer length and function of protomers in heteromers, with spacers ranging from 6 to 21 atoms. Given that our data is consistent with efficient bridging in the range of 18-22 atoms.29-36, 40-43 For the present series, we have synthesized bivalent ligands that contain 2-, 6-, 15-, 19- and 20-atoms (1-5, respectively), with the expectation that 4 and/or 5 would be capable of bridging the protomers. Monovalent mu agonist 9 and CB1 antagonist 10 served as controls in our studies. The bivalent ligands 1-5 consisted of a mu agonist tethered to a CB1 antagonist pharmacophore that was modeled after 7 with a minor modification of the piperidine ring based upon reported SAR studies.44
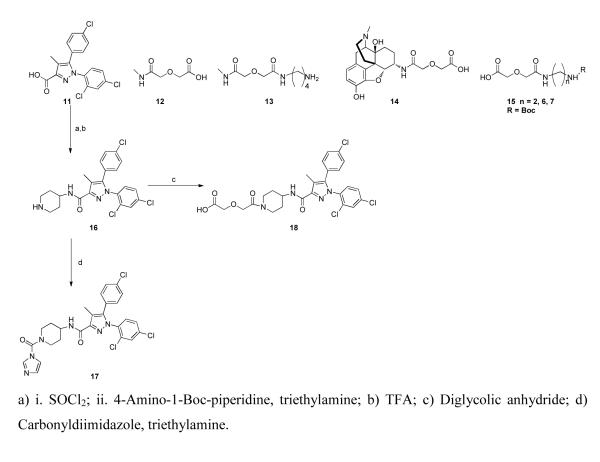
The choice of 16 as a CB1 antagonist pharmacophore and intermediate in the synthesis was based upon reported SAR studies of 7.44 This intermediate was prepared from 1145 and converted to 18 with diglycolic anhydride or activated with carbonyldiimidazole to afford 17 (Scheme 1). Intermediates 12-15 are described in the literature.35 Intermediate 16 is a modified analog of 8 whose binding affinity and CB2/CB1 selectivity is comparable to SR141716 (7).
Scheme 1.
Intermediates for synthesis of bivalent ligands 1-5 and monovalent ligands 9 and 10
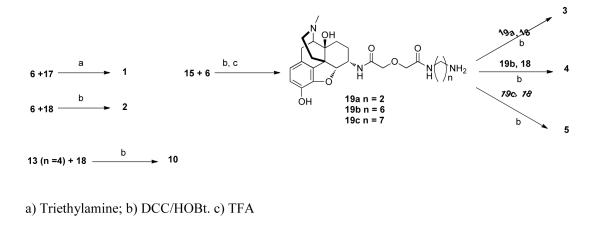
The synthesis of target compounds 1-5 and 10 is outlined in Scheme 2. Bivalent ligands 1 and 2 were prepared by reacting 6 with CB1 antagonist intermediates 17 and 18, respectively. The intermediates (19a, 19b, 19c) for the synthesis of 3-5 were obtained by coupling 6 with the spacer components 15a, 15b, 15c (Scheme 1), respectively. Condensation of 19a with 18 afforded 3. Intermediates 19b and 19c gave rise to 4 and 5, respectively, via standard coupling procedure. The monovalent ligand 10 was prepared by coupling 13 (n = 4) with 18. The monovalent ligand 9 was prepared previously.46
Scheme 2.
Synthesis of target compounds (1-5, 10)
Biological studies
Immunofluorescence
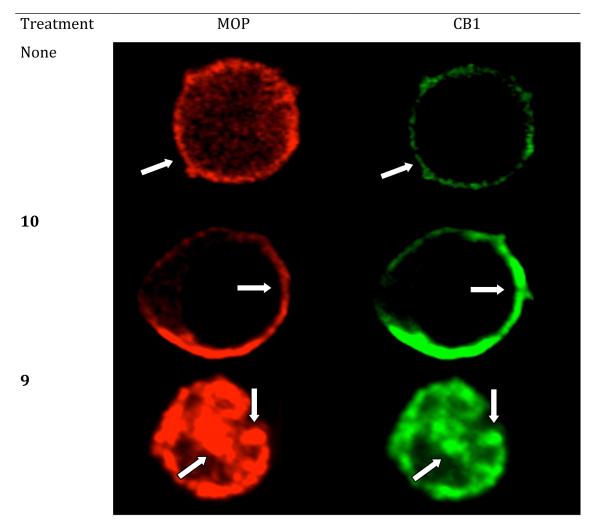
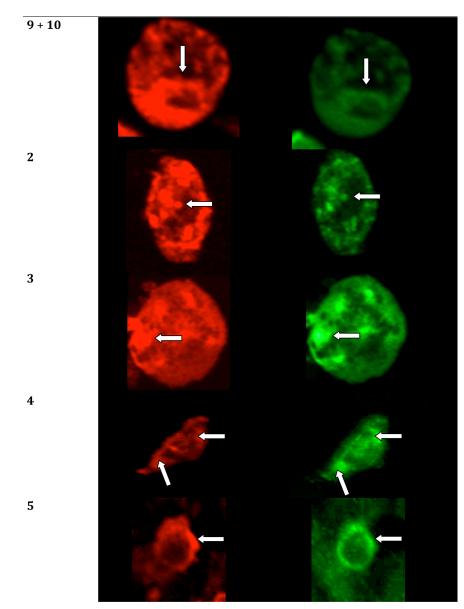
Transiently co-expressing MOP and CB1 receptors in HEK-293 cells were treated with immunofluorescent antibodies to image the receptors after treatment with monovalent (9, 10) and bivalent (2-5) ligand. The present study was based on prior imaging studies that have revealed a lack of internalization of co-expressing MOP and DOP receptors in HEK-293 cells upon treatment with a MOP agonist/DOP antagonist bivalent ligand (MDAN-21) which is capable of bridging neighboring protomers. This was was in contrast to its homologue (MDAN-16) with a short spacer or a combination of monovalent MOP agonist/DOP antagonist ligands.49 Thus, the internalization assay represents a valuable correlate for evaluation of agonist-antagonist bivalent ligands bridging GPCR heteromers.
Under basal conditions (no treatment) or in the presence of monovalent CB1 antagonist 10, MOP and CB1 receptors were localized exclusively on the plasma membrane. (Fig. 2) However, treatment with either monovalent agonist 9, a mixture of monovalent agonist and antagonist (9+10), or bivalent ligands (2, 3) with relatively short spacers, resulted in robust receptor clustering and endocytosis. A significant level of fluorescence also was observed in the cytosol when the combination of both monovalent ligands 9 and 10 were used and the internalization was similar to that observed for control ligand 9 alone. Treatment with the monovalent CB1 antagonist alone produced no internalization. Ligand 4 did not produce as intense co-internalization as shorter bivalent ligands, especially at MOP receptor. Significantly, no co-internalization of mu and CB1 receptors was induced by the longest bivalent ligand 5 as both receptors remained located on the plasma membrane. These data are consistent with the idea that 5 with a 20 atom spacer bridges neighboring protomers. The specific number of atoms required for bridging for a particular bivalent series is dependent on both the constitution of the antagonist pharmacophore and the bound protomer.
Figure 2.
Single cell high-magnification images of double-labeling immunofluorescence for co-expressed MOP and CB1 receptors on HEK-293 cells. The identical cells are shown labeled for MOP (red fluorescence) and CB1 (green fluorescence) receptors. Arrows depict profiles double-labeled for MOP and CB1.
Pharmacological evaluation
The in vivo profiles of bivalent ligands with 2-, 6-, 15-, 19- and 20-atom spacers (1-5) and monovalent ligands (9, 10) were determined using the mouse tail-flick assay. The monovalent ligands were administered either singly or in combination. All compounds were evaluated for antinociception and tolerance after intracerebroventricular (i.c.v.) or intrathecal (i.t.) injection. These data are summarized in Table 1. For evaluation of tolerance, the ED80–90 dose was measured on day one and compared to the same dose measured 24 hours later in the same mice. With the exception of the monovalent opioid agonist 9 and the bivalent ligand 1, the bivalent compounds (2-5) and combination of monovalent ligands 9 and 10 were devoid of tolerance. Tolerance occurred only when the agonist 9 was applied alone36 or when 1 was administered i.c.v.
Table 1.
Antinociception and 24 hour tolerancea evaluation after i.c.v. and i.t. administration of compounds 1-5 and 9-10 in ICR-CD1 mice
| Cmpd | I.C.V. | I.T. | Ratio i.c.v./i.t. |
||
|---|---|---|---|---|---|
| ED50 (95% C.I.) pmol/mouse |
24hr tolerance | ED50 (95% C.I.) pmol/mouse |
24hr tolerance | ||
| 1 | 1219 (859- 1730) |
Day 1: 64.27 ± 6.55 Day 2: 33.85 ± 11.38 Tolerance |
559 (372- 840) |
Day 1: 73.55 ± 10.93 Day 2: 66.01 ± 6.81 No Tolerance |
2.18 |
| 2 | 523 (223-1228) |
Day 1: 77.72 ± 9.78 Day 2: 63.70 ± 16.48 No Tolerance |
27 (13-54) |
Day 1: 81.72 ± 9.48 Day 2: 73.41 ± 11.49 No Tolerance |
19.48 |
| 3 | 55 (37-82) |
Day 1: 83.47 ± 11.59 Day 2: 66.99 ± 9.55 No Tolerance |
42 (31-58) |
Day 1: 98.33 ± 1.79 Day 2: 72.5 ± 11.22 No Tolerance |
1.31 |
| 4 | 189 (132-270) |
Day 1: 95.43 ± 2.93 Day 2: 86.33 ± 7.88 No Tolerance |
19 (11-34) |
Day 1: 78.51 ± 10.44 Day 2: 79.58 ± 9.82 No Tolerance |
9.86 |
| 5 | 9 (6- 12) |
Day 1b: 89.75 ± 10.95 Day 3: 72.81 ± 12.69 No Tolerance |
5 (3- 8) |
Day 1: 92.20 ± 8.34 Day 2: 87.91 ± 6.76 No Tolerance |
1.8 |
| 9 | 108 (83 - 141) |
Day 1: 82.15 ± 9.67 Day 2: 24.14 ± 11.92 Tolerance |
106 (41 - 273) |
Day 1: 75.98 ± 12.73 Day 2: 39.54 ± 14.28 Tolerance |
1.01 |
| 10 | NSc | NAd | NS | NA | |
| 9+10 e | 78 (55-110) |
Day 1: 68.84 ± 11.94 Day 2: 43.92 ± 15.20 No Tolerance |
130 (97-173) |
Day 1: 78.00 ± 11.62 Day 2: 74.83 ± 8.45 No Tolerance |
0.60 |
24 hour tolerance was calculated using the highest dose of the dose-response curve on day 1 and repeated on day 2. If there was no significant difference between the two days the animals were said to be not tolerant. Compounds 9+10 (equimolar dose), 1-5 were administered at 2500, 250, 1000 and 125 pmol per mouse.
As there was 47% antinociception 24 hours after the initial 15 pmol/mouse (i.c.v.) dose, a 24 hour tolerance test could not be completed. However, after 48 hours the antinociception had returned to baseline and, at this time tolerance was measured by injection of a subsequent 15 pmol/mouse.
NS: not significant; compound 10 did not show any antinociceptive activity (only 20-30%) when given at 250 or 2500 pmol per mouse.
Not applicable.
Doses were based on a 1:1 mixture of compounds 9+10.
The monovalent CB1 antagonist 10, like its parent compound 7 (SR141716),44a did not produce antinociception even at a relatively high dose (2.5 nmol/mouse). There appeared to be no significant difference in potency after acute co-administration of an equimolar dose ratio of 9 and antagonist 10 when compared to that of 9 alone. However, in contrast to 9 alone, the co-administration with CB1 antagonist did not produce 24 hour tolerance either by the i.c.v or i.t. routes (Table 1). This was consistent with the absence of tolerance of bivalent ligands 2-5, presumably due to the presence of the CB1 antagonist pharmacophore.
The i.c.v. antinociception SAR profile of bivalent ligands 1-5 was somewhat different from that obtained after i.t. administration. By the i.c.v. route, a mixture of 9+10 was significantly more potent than 1 or 4, whereas this mixture was clearly less potent than 5. When administered i.t., bivalents 2-5 were significantly more potent than 9+10. Upon i.c.v. administration, 5 (20-atom spacer) exhibited a substantial 20-fold potency increase relative to its lower homologue 4 with a 19-atom spacer. By comparison, there was only a 4-fold increase by the i.t. route. Significantly, 5 not only is the most potent member of the series, but was still active (47%) 24 hours after i.c.v. admininistration. This long duration of action led us to postpone tolerance measurement to 48 hours as mentioned in Table 1.
Discussion
A key side effect of morphine and related mu agonists in the pharmacotherapy of chronic pain is the development of tolerance. Prior studies12-18 have shown there is pharmacologic interaction between MOP and cannabinoid CB1 receptors. In view of evidence for heteromeric MOP-CB1 receptors in cultured cells, and the report that i.t. co-administration of morphine with a CB1 antagonist prevents tolerance in rats, 19, 25, 26 we have investigated whether this effect is mediated via the physical association of MOP and CB1 receptors or through other mechanisms.
Our approach to addressing this question involved the design and synthesis of bivalent ligands that contain mu opioid agonist and CB1 antagonist pharmacophores. As we have reported that spacers in the range of 18-22 atoms can lead to bridging of protomers in a variety of heteromers containing opioid receptors in cultured cells,32, 33, 49 , 50 such a bivalent ligand could be useful in selectively targeting a MOP-CB1 heteromer to determine if opioid tolerance is affected.
Accordingly, we have synthesized a series of bivalent ligands containing both mu agonist and CB1 antagonist pharmacophores that are connected by shorter spacers (1-3) or longer spacers (4, 5). Control monovalent ligands 9 and 10 also were prepared. Bivalent ligands 1-3 would be expected to interact univalently with either MOP or CB1 receptors, whereas 4 and 5 containing 19- and 20-atom spacers, respectively, have been reported to be in the bridging range of protomers in other heteromers. 29-36, 49, 50
An immunocytochemical-derived correlate for establishing bridging of protomers by a bivalent ligand containing mu agonist and delta antagonist pharmacophores in MOR-DOR heteromer in HEK-293 cells has been employed.49 In that particular study, it was determined in HEK293 cells that bivalent ligands with a 21- and 16-atom spacers (MDAN21 and MDAN16) exhibit different trafficking, in that receptors that are not bridged undergo agonist-induced endocytosis while those that are bridged remain on the cell surface.48, 49 Thus, MDAN21 prevented internalization whereas MDAN16-treated cells exhibited endocytosis. Similar studies performed on HEK-293 cells co-expressing MOP and CB1 receptors are consistent with the view that bridging of protomers takes place only with compound 5 (20-atom spacer), as no significant receptor internalization was observed. On the other hand, bivalent ligands with 6-, 15- and 19-atom spacers (2, 3, and 4, respectively) induced robust internalization, as did monovalent mu agonist 9, or a mixture of 9 and monovalent CB1 antagonist 10. (Fig. 2)
The evaluation of antinociception and tolerance in mice using two routes of administration (i.c.v. and i.t.) revealed that bivalent ligands 2-5 and a mixture of monovalent mu agonist 9 and CB1 antagonist 10 were devoid of tolerance. These data afforded results that are consistent with pharmacologic interaction between MOP and CB1 receptors. It is noteworthy that bivalent ligand 1 with the shortest (2-atom) spacer possessed substantially lower potency with tolerance (Table 1) relative to other bivalent ligands 2-5 when administered i.c.v. Since 1 possesses a two-atom spacer, it is likely that the CB1 pharmacophore’s antagonist activity has been compromised due to its proximity to the opioid pharmacophore. In the absence of CB1 antagonist activity, 1 would therefore produce tolerance. The reduced antinociception is likely due to steric hindrance of the opioid pharmacophore at the MOP receptor by the CB1 pharmacophore.
The finding that bivalent ligands 2-5, whose spacers range from 6 to 20 atoms, and a mixture of monovalents (9 + 10) are all devoid of tolerance suggests that the ability of CB1 antagonist to reduce or prevent opioid-induced tolerance is not necessarily mediated via a MOP-CB1 heteromer. On the other hand, because the most potent bivalent ligand 5 is capable of bridging bridging such a heteromer in HEK293 cells, it cannot be excluded as a target by 5.
It is perhaps significant that 5 is substantially more potent than its lower homologue 4 by a factor of 20 when given supraspinally. On the other hand, 5 is more potent than 4 only by a factor of 4 following the i.t. route. The larger potency difference i.c.v. suggests that there may be more MOP-CB1 heteromer supraspinally. This would be consistent with our observation that the day 2 tolerance test could not be conducted because of residual antinociception (~47%). The possible supraspinal localization of MOP-CB1 heteromer is a departure from MOP-DOP36 and DOP-KOP30, 51 heteromers that appear to be localized in the cord.
In conclusion, we have obtained immunofluorescence evidence in HEK293 cells that support the bridging of MOP and CB1 protomers in a heteromer by bivalent ligand 5 that contains a mu agonist linked to a CB1 antagonist via a 20-atom spacer. Lower homologues with 19 or fewer atoms connecting the pharmacophores do not share this property. Bivalent ligand 5 possessed the highest antinociceptive potency when administered either i.c.v. or i.t.. It was found that 5 and lower homologue bivalent ligands 2-4 that do not bridge MOP-CB1 heteromer, as well as a mixture of monovalent ligands (mu agonist 9 and CB1 antagonist 10) all were free of opioid tolerance. Thus, it appears that MOP-CB1 heteromer may not be an important exclusive target for inhibition of opioid tolerance by co-administration. These results further support the synergestic effects between the opioid and cannabinoid systems and the possible benefit of using a CB1 antagonist component to oppose the development of tolerance induced by opioids.
Experimental section
All commercial reagents and anhydrous solvents that were purchased from vendors were used without further purification or distillation. Analytical thin-layer chromatography (TLC) was performed on EM Science silica gel 60 F254 (0.25 mm). Plates were visualized by UV light, iodine vapor, or ninhydrin solution. Flash column chromatography was performed on Fisher Scientific silica gel (230 – 400 mesh). Melting points were determined on a Thomas-Hoover melting point apparatus and are uncorrected. 1H NMR spectra and 13C NMR spectra were taken on a Bruker Avance 400 MHz instruments and calibrated using an internal reference. Chemical shifts are expressed in ppm and coupling constants (J) are in hertz (Hz). Peak multiplicities are abbreviated: broad, br; singlet, s; doublet, d; triplet, t; and multiplet, m. ESI mode mass spectra were recorded on a BrukerBioTOF II mass spectrometer. Elemental analyses were performed by M-H-W Laboratories, Phoenix, AZ. Analytical data confirmed the purity of the products was ≥ 95%.
General procedure for DCC/HOBt coupling
DCC (37 mg, 0.18 mmol), carboxylic acid 16 (70 mg, 0.12 mmol), and HOBt (22 mg, 0.16 mmol) were dissolved in 5 mL of anhydrous DMF. The solution was cooled to 0°C and placed under a nitrogen atmosphere. After 15 minutes at 0°C, amine (0.11 mmol) was added. The solution was sealed under a nitrogen atmosphere and was allowed to warm and to stir at room temperature overnight. The reaction mixture was filtered in order to remove dicyclohexylurea. The filtrate was poured into water (10x initial volume of DMF) and extracted with ethyl acetate. The combined organic layers were dried on magnesium sulfate, filtered and concentrated under reduced pressure. The residue was then purified by flash chromatography. The title compound was then subsequently converted into the HCl salt for biological testing.
4-(5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamido)-N-((4αS,7S,7αR,12βS)-4α,9-dihydroxy-3-methyl-2,3,4,4α,5,6,7,7α-octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7-yl)piperidine-1-carboxamide; (1)
Combining amine 6 (85 mg, 0.28 mmol), compound 17 (172 mg, 0.30 mmol) and triethylamine (48 μL, 0.34 mmol) in DCM gave the target compound which was purified by flash chromatography [MeOH/DCM/NH4OH=3/96/1] to provide 1 as a white solid. Yield: 188 mg, 85%.
1H NMR (CD3OD) δ 0.97 (m, 1H) ; 1.34-1.48 (m, 4H) ; 1.68-1.80 (m, 3H) ; 1.94 (m, 1H) ; 2.03 (m, 2H) ; 2.14 (s, 3H) ; 2.59 (m, 2H) ; 2.85 (s, 3H) ; 3.07-3.13 (m, 2H) ; 3.16-3.22 (m, 2H) ; 3.13 (D, 1H, J= 8.9 Hz) ; 3.23 (m, 1H) ; 3.83 (m, 1H) ; 4.14 (m, 1H) ; 4.35 (m, 1H) ; 4.49 (m, 1H) ; 4.64 (m, 1H) ; 6.64 (D, 1H, J= 8.2 Hz) ; 6.73 (D, 1H, J= 8.2 Hz) ; 7.21 (D, 2H, J= 8.5 Hz) ; 7.38 (D, 2H, J= 8.4 Hz) ; 7.45 (m, 2H) ; 7.61 (d, 1H, J= 2.2 Hz) ; 13C NMR δ 8.84; 22.02; 30.62; 30.75; 33.57; 42.08; 46.50; 47.19; 48.27; 55.83; 61.54; 64.27; 68.16; 71.05; 82.50; 90.38; 117.08; 119.36; 120.66; 123.34; 126.93; 128.37; 128.57; 129.30; 129.97 (2x); 130.12; 131.18; 132.29 (2x); 132.50; 134.34; 136.27; 137.35; 140.41; 143.86; 147.32; 147.78; 159.57; 165.25. Anal. Calcd. for C40H41Cl3N6O7 : C, 60.65 ; H, 5.22 ; N, 10.61. Found : C, 60.78 ; H, 5.23 ; N, 10.57. ESI-TOF MS calculated for C40H41Cl3N6O5, m/z 792.212, found 793.272 (MH)+
5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-N-(1-(2-(2-(((4αS,7S,7αR,12βS)-4α,9-dihydroxy-3-methyl-2,3,4,4α,5,6,7,7α-octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7-yl)amino)-2-oxoethoxy)acetyl)piperidin-4-yl)-4-methyl-1H-pyrazole-3-carboxamide; (2)
Compound 2 was prepared in a similar fashion as described above; combining α-oxymorphamine (6) (33 mg, 0.11 mmol), acid 18 (70 mg, 0.12 mmol) ; DCC (37 mg, 0.18 mmol), and HOBt (22 mg, 0.16 mmol) gave the target compound which was purified by flash chromatography [MeOH/DCM/NH4OH=2/97/1] to provide 2 as a white solid. Yield: 74 mg, 78%.
1H NMR (CD3OD) δ 1.01 (m, 1H) ; 1.25-1.36 (m, 4H) ; 1.41-1.57 (m, 4H) ; 1.80 (m, 1H) ; 2.01 (m, 2H) ; 2.17 (s, 3H) ; 2.25 (m, 2H) ; 2.35 (s, 3H) ; 2.43 (m, 1H) ; 2.64 (m, 1H) ; 2,81 (m, 1H) ; 2.97 (m, 1H) ; 3.13 (D, 1H, J= 8.9 Hz) ; 3.23 (m, 1H) ; 3.98-4.09 (m, 4H) ; 4.26 (m, 1H) ; 4.63 (m, 1H) ; 4.70 (m, 1H, CONH) ; 6.66 (D, 1H, J= 8.2 Hz) ; 6.71 (D, 1H, J= 8.2 Hz) ; 7.07 (D, 2H, J= 8.5 Hz) ; 7.11 (D, 1H, J= 8.4 Hz) ; 7.25 (Dd, 1H, J= 8.4 Hz, J= 2.2 Hz) ; 7.32 (D, 2H, J= 8.6 Hz) ; 7.45 (d, 1H, J= 2.2 Hz) ; 7.67 (m, 1H, CONH) ; Anal. Calcd. for C43H45Cl3N6O7 : C, 59.76 ; H, 5.25 ; N, 9.72. Found : C, 59.88 ; H, 5.13 ; N, 9.77. ESI-TOF MS calculated for C43H45Cl3N6O7, m/z 864.212, Found 865.372 (MH)+
5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-N-(1-(14-(((4αS,7S,7αR,12βS)-4α,9-dihydroxy-3- methyl-2,3,4,4α,5,6,7,7α-octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7-yl)amino)-5,10,14-trioxo-3,12-dioxa-6,9-diazatetradecan-1-oyl)piperidin-4-yl)-4-methyl-1H-pyrazole-3-carboxamide; (3)
Compound 3 was prepared in a similar fashion as described above; combining amine 19a (51 mg, 0.11 mmol), acid 18 (70 mg, 0.12 mmol) ; DCC (37 mg, 0.18 mmol), and HOBt (22 mg, 0.16 mmol) gave the target compound which was purified by flash chromatography [MeOH/DCM/NH4OH=2/97/1] to provide 3 as a white solid. Yield: 73 mg, 65%.
1H NMR (CD3OD) δ 0.97 (m, 1H) ; 1.25-1.36 (m, 5H) ; 1.41-1.57 (m, 4H) ; 1.80 (m, 1H) ; 2.01-2.04 (m, 2H) ; 2.16 (s, 3H) ; 2.25-2.29 (m, 2H) ; 2.35 (s, 3H) ; 2.43 (m, 1H) ; 2.64 (m, 1H) ; 2,81 (m, 1H) ; 2.97 (m, 1H) ; 3.11 (D, 1H, J= 8.9 Hz) ; 3.14-3.28 (m, 4H) ; 4.00-4.08 (m, 8H) ; 4.24 (m, 1H) ; 4.59 (m, 1H) ; 6.54 (D, 1H, J= 8.2 Hz) ; 6.73 (D, 1H, J= 8.2 Hz) ; 7.04 (D, 2H, J= 8.5 Hz) ; 7.14 (D, 1H, J= 8.4 Hz) ; 7.21 (Dd, 1H, J= 8.4 Hz, J= 2.2 Hz) ; 7.35 (D, 2H, J= 8.6 Hz) ; 7.41 (d, 1H, J= 2.2 Hz) ; 7.86 (m, 1H, CONH) ; Anal. Calcd. for C49H55Cl3N8O10 : C, 57.56 ; H, 5.42 ; N, 10.96. Found : C, 57.72 ; H, 5.43 ; N, 10.87. ESI-TOF MS calculated for C49H55Cl3N8O10 , m/z 1022.368, found 1023.276 (MH)+
5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-N-(1-(18-(((4αS,7S,7αR,12βS)-4α,9-dihydroxy-3-methyl-2,3,4,4α,5,6,7,7α-octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7-yl)amino)-5,14,18-trioxo-3,16-dioxa-6,13-diazaoctadecan-1-oyl)piperidin-4-yl)-4-methyl-1H-pyrazole-3-carboxamide; (4)
Compound 4 was prepared in a similar fashion as described above; combining amine 19b (57 mg, 0.11 mmol), acid 18 (70 mg, 0.12 mmol) ; DCC (37 mg, 0.18 mmol), and HOBt (22 mg, 0.16 mmol) gave the target compound which was purified by flash chromatography [MeOH/DCM/NH4OH=2/97/1] to provide 4 as a white solid. Yield: 67 mg, 57%.
1H NMR (CD3OD) δ 0.88 (m, 1H) ; 1.24-1.34 (m, 9H) ; 1.40-1.59 (m, 8H) ; 1.79 (m, 1H) ; 1.99-2.02 (m, 2H) ; 2.16 (s, 3H) ; 2.28-2.24 (m, 2H) ; 2.36 (s, 3H) ; 2.45 (m, 1H) ; 2.61 (m , 1H) ; 2.86-2.91 (m, 2H) ; 3.09 (D, 1H, J= 8.8 Hz) ; 3.14-3.18 (m, 4H) ; 3.99-4.07 (m, 8H) ; 4.20 (m, 1H) ; 4.25 (m, 1H, CONH) ; 4.56 (m, 1H) ; 4.60 (m, 1H, CONH) ; 6.53 (D, 1H, J= 8.2 Hz) ; 6.72 (D, 1H, J= 8.2 Hz) ; 7.04 (D, 2H, J= 8.5 Hz) ; 7.15 (D, 1H, J= 8.4 Hz) ; 7.25 (Dd, 1H, J= 8.4 Hz, J= 2.2 Hz) ; 7.29 (D, 2H, J= 8.6 Hz) ; 7.44 (d, 1H, J= 2.2 Hz) ; 7.49 (m, 1H, CONH) ; Anal. Calcd. for C43H45Cl3N6O7 : C, 59.02 ; H, 5.89 ; N, 10.39. Found : C, 59.18 ; H, 5.73 ; N, 10.24. ESI-TOF MS calculated for C53H63Cl3N8O10 , m/z 1078.474, found 540.171 (M/2H)+, 1079.366 (MH)+
5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-N-(1-(19-(((4αS,7S,7αR,12βS)-4α,9-dihydroxy-3-methyl-2,3,4,4α,5,6,7,7α-octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7-yl)amino)-5,15,19-trioxo-3,17-dioxa-6,14-diazanonadecan-1-oyl)piperidin-4-yl)-4-methyl-1H-pyrazole-3-carboxamide; (5)
Compound 5 was prepared in a similar fashion as described above; combining amine 19c (65 mg, 0.12 mmol), acid 18 (77 mg, 0.13 mmol) ; DCC (41 mg, 0.20 mmol), and HOBt (23 mg, 0.17 mmol) gave the target compound which was purified by flash chromatography [MeOH/DCM/NH4OH=7/92/1] to provide 5 as a clear oil. Yield: 28 mg, 22%.
1H NMR (CD3OD) δ 0.92 (m, 1H) ; 1.28-1.43 (m, 11H) ; 1.47-1.62 (m, 8H) ; 1.83 (m, 1H) ; 2.07-2.17 (m, 2H) ; 2.18 (s, 3H) ; 2.39-2.42 (m, 2H) ; 2.46 (s, 3H) ; 2.51 (m, 1H) ; 2.57 (m , 1H) ; 2.89-2.95 (m, 2H) ; 3.11 (D, 1H, J= 8.8 Hz) ; 3.15-3.21 (m, 4H) ; 4.01-4.10 (m, 8H) ; 4.23 (m, 1H) ; 4.63 (m, 1H) ; 4.71 (m, 1H, CONH) ; 6.51 (D, 1H, J= 8.2 Hz) ; 6.69 (D, 1H, J= 8.2 Hz) ; 7.04 (D, 2H, J= 8.5 Hz) ; 7.13 (D, 1H, J= 8.4 Hz) ; 7.19 (Dd, 1H, J= 8.4 Hz, J= 2.2 Hz) ; 7.23 (D, 2H, J= 8.6 Hz) ; 7.41 (d, 1H, J= 2.2 Hz) ; 7.57 (m, 1H, CONH) ; Anal. Calcd. for C54H65Cl3N8O10 : C, 59.37 ; H, 6.00 ; N, 10.26. Found : C, 59.51 ; H, 5.97 ; N, 10.31. ESI-TOF MS calculated for C54H65Cl3N8O10 , m/z 1092.501, found 1093.671
5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(1-(3,7,14-trioxo-5,16-dioxa-2,8,13-triazaoctadecan-18-oyl)piperidin-4-yl)-1H-pyrazole-3-carboxamide; (10)
Compound 10 was prepared in a similar fashion as described above; combining amine 13 (n = 4) (24 mg, 0.11 mmol), acid 18 (70 mg, 0.12 mmol) ; DCC (37 mg, 0.18 mmol), and HOBt (22 mg, 0.16 mmol) gave the target compound which was purified by flash chromatography [MeOH/DCM= 1/99] to provide 10 as a white solid. Yield. 36 mg, 42%.
1H NMR (CD3OD) δ 1.51-1.55 (m, 7H) ; 1.98 (m, 2H) ; 2,17 (s, 3H) ; 2.79 (s, 3H) ; 2.96 (m, 1H) ; 3.10 (m, 2H) ; 3.24-3.30 (m, 4H) ; 4.00-4.02 (m, 8H) ; 4.13 (m, 1H), 4.36 (d, 1H, J= 7.3 Hz) ; 4.70-4.73 (m, 2H, NH) ; 7.06 (D, 2H, J= 8.5 Hz) ; 7.16 (D, 1H, J= 8.4 Hz) ; 7.25 (Dd, 1H, J= 8.4 Hz, J= 2.2 Hz) ; 7.34 (D, 2H, J= 8.6 Hz) ; 7.45 (d, 1H, J= 2.2 Hz) ; Anal. Calcd. for C35H42Cl3N7O7 : C, 53.96 ; H, 5.43 ; N, 12.58. Found : C, 53.88 ; H, 5.52 ; N, 12.67. ESI-TOF MS calculated for C35H42Cl3N7O7 , m/z 779.110, Found 780.284 (MH)+
5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-4-yl)-1H-pyrazole-3- carboxamide (16)
A mixture of the carboxylic acid 11 (1.14 g, 3 mmol) and thionyl chloride (0.8 mL, 11 mmol) in 20 mL of toluene was refluxed for 3 h and then evaporated to dryness under reduced pressure. The residue was taken up in 20 mL of toluene and the solvent was evaporated again under reduced pressure to give the crude acid chloride, which was dissolved in 15 mL of dry dichloromethane. This solution was added drop wise to a solution of N-Boc-4-aminopiperidine (901 mg, 4.5 mmol) and triethylamine (620 μL, 4.5 mmol) in 10 mL of dichloromethane, cooled to 0°C. After stirring at room temperature for 16 h, the reaction mixture was added to brine (30 mL) and extracted with dichloromethane. The organic layer was separated, dried (magnesium sulphate) and evaporated under reduced pressure. The residue was then taken up in DCM and TFA was added (20% volume). The mixture was stirred 16 h at room temperature. The solvent was evaporated under reduced pressure and the residue was co-evaporated with toluene (2X). The crude mixture was then purified by flash chromatography (EA) to afford compound 16 as white foam. Yield: 1.14 g, 82% (overall).
1H NMR (CDCl3) δ : 1.41 (m, 2H) ; 1. 52 (m, 2H) ; 1.93 (m, 2H) ; 2.18 (s, 3H) ; 2.95 (m, 2H) ; 3.20 (m, 1H) ; 4.29 (d, 1H, NH, J= 7.4 Hz) ; 4.65 (d, 1H, NH, J= 7.2 Hz) ; 7.07 (d, 2H, J= 8.5 Hz) ; 7.15 (d, 1H, J= 8.4 Hz) ; 7.23 (dd, 1H, J= 8.4 Hz, J= 2.2 Hz) ; 7.39 (D, 2H, J= 8.6 Hz) ; 7.45 (d, 1H, J= 2.2 Hz) ; 13C NMR (CDCl3) δ 163.1; 143.0; 138.5; 136.0; 135.2; 135.1; 133.1; 130.9 (x2); 130.1; 129.9; 128.7 (x2); 127.8; 12 7.2; 118.2; 56.2; 31.8 (x2); 25.8; 23.5; 8.7. mp 162-163°C (decomposition); ESI-TOF MS calculated for C22H21Cl3N4O , m/z 463.787, found 464.705 (MH)+
N-(1-(1H-Imidazole-1-carbonyl)piperidin-4-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (17)
To a solution of piperidine 16 (200 mg, 0.43 mmol) dissolved in DCM with triethylamine (90 μL, 0.64 mmol), carbonyldiimidazole was added dropwise (83 mg, 0.52 mmol) at room temperature. The mixture was stirred overnight and was concentrated to dryness. The residue was purified by flash chromatography [MeOH/DCM=2/98] to provide 17 as a white solid. Yield: 209 mg, 87%.
1H NMR (CDCl3) 1.80–1.87 (m, 2H) ; 2.25-2.29 (m, 5H with s, 3H at 2.29) ; 3.10 (t, 1H, J= 11.9 Hz) ; 3.41 (t, 1H, J= 12.2 Hz) ; 4.30 (m, 1H) ; 4.50 (d, 1H, J= 13.4 Hz) ; 4.95 (d, 1H, J= 13.3 Hz) ; 7.04 (D, 2H, J= 8.5 Hz) ; 7.15 (D, 1H, J= 8.4 Hz) ; 7.18 (s, 1H) ; 7.25 (Dd, 1H, J= 8.4 Hz) ; 7.18 (s, 1H) ; 7.25 (Dd, 1H, J= 8.4 Hz, J= 2.2 Hz) ; 7.29 (D, 2H, J= 8.6 Hz) ;7.52 (s, 1H) ; 8.49 (s, 1H) ; 8.77 (d, 1H, J= 2.3 Hz) ; 13C NMR (CDCl3) δ 8.89 ; 31.50. 32.76 ; 41.53 ; 46.52 ; 48.73 ; 115.97 ; 116.72 ; 121.90 (2x) ; 126.96 ; 127.90 ; 128.99 (2x) ; 129.33 ; 129.57 ; 130.60 (2x) ; 132.91 ; 135.69 ; 135.89 ; 136.32 ; 142.12 ; 146.39 ; 148.81 ; 163.78. mp 189-191°C. ESI-TOF MS calculated for C26H23Cl3N6O2 , m/z 557.859, Found 558.913 (MH)+
2-(2-(4-(5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamido)piperidin-1-yl)-2-oxoethoxy)acetic acid (18)
To a solution of piperidine 16 (278 mg, 0.6 mmol) dissolved in DCM with pyridine (60 mL, 0.72 mmol), diglycolic anhydride (70 mg, 0.6 mmol) was added dropwise. The mixture was stirred at room temperature overnight. After disappearance of starting material, the solvent was concentrated to dryness; the residue was co-evaporated with toluene (3X) to afford 18 as a white solid. Yield: 347 mg, quantitative. Compound 18 was used without further purification.
1H NMR (CDCl3) δ : 1.59 (m, 2H) ; 2.03 (m, 2H) ; 2.18 (s, 3H) ; 3.06 (m, 1H) ; 3.32 (m, 1H) ; 4.07-4.24 (m, 5H) ; 4.33 (m, 1H) ; 4.65 (m, 1H) ; 7.07 (D, 2H, J= 8.4 Hz) ; 7.16 (D, 1H, J= 8.3 Hz) ; 7.24 (Dd, 1H, J= 8.4 Hz, J= 2.2 Hz) ; 7.29 (D, 2H, J= 8.6 Hz) ; 7.43 (d, 1H, J= 2.2 Hz) ; 13C NMR (CDCl3) δ 171.9; 169.5; 163.7; 143.0; 138.8; 136.7; 136.6; 135.3; 133.7; 130.6 (x2); 130.5; 130.3; 128.9 (x2); 127.8; 127.1; 118.2; 72.3; 68.4; 57.2; 46.28 (x2); 32.9; 31.3; 8.9; mp 227-228°C; ESI-TOF MS calculated for C26H25Cl3N4O5 , m/z 579.860, found 578.673(MH)−
Biology
Immunofluorescence protocol
To determine the effects of the MOP-CB1 bivalent ligands on the trafficking of MOP and CB1 receptors, we utilized the immunofluorescence technique49 in HEK-293 cells transiently expressing the receptors. HEK-293 cells were transfected with cDNA for hMOPR and hCB1 (16 μg/ 2 million cells) and seeded 24 hours later into 8-well chamber slides. After allowing for a further 24 hours for the cells to settle and equilibrate, they were incubated with the different ligands (1 μM) for 30 minutes. The cells were then washed, fixed with 4% paraformaldehyde and incubated with the mu (Neuromics Inc.) and CB1 selective primary antibodies (R&D Systems) overnight at 4°C. The cells were then washed the next day and stained with respective fluorescent secondary antibodies (red for MOP, green for CB1). Images were acquired using a Olympus BX10 confocal microscope and analyzed using ImageJ software (NIH).
Animals
Male ICR-CD1 mice (17 – 25g; Harlan, Madison, WI), are housed in groups of 8 in a temperature- and humidity-controlled environment with unlimited access to food and water. They are maintained on a 12 h light/dark cycle. All experiments are approved by the Institutional Animal Care and Use Committee of the University of Minnesota (Minneapolis, MN).
Antinociceptive Testing
The tail flick assay described by D’Amour and Smith52 and modified by Dewey et. al. was used to test for antinociception.53 For the measurement of the tail-flick latency, mice are held gently in one hand with the tail positioned in the apparatus (Tail Flick Analgesia Meter, Columbus Instruments, Columbus, Ohio) for radiant heat stimulus. The tail-flick response is elicited by applying radiant heat to the dorsal side of the tail. The intensity of the heat is set so that the mouse flicks its tail within 2 to 3 s. The test latency is measured before drug treatment (control) and again after the drug treatment (test) at the peak time of the compound, a 10 s maximum cut-off time is used to prevent damage to the tail. Antinociception is quantified according to the method of Harris and Pierson54 as the percent maximal possible effect (%MPE) which is calculated as: %MPE = (Test - Control/10 – Control) × 100. At least three groups of eight to ten mice were used for each dose response curve, and each mouse is used only once. ED50 values with 95% confidence intervals (C.I.) are computed with GraphPad Prism 4 by using nonlinear regression methods.
Acknowledgements
This research was supported by National Institute on Drug Abuse research grant DA01533, We thank Prof. Alex Makriyannis for preliminary binding data on CB1 antagonist analogues.
References
- 1.Vigano D, Rubino T, Parolaro D. Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacol., Biochem. Behav. 2005;81:360–368. doi: 10.1016/j.pbb.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 2.Mansour A, Khachaturian H, Lewis M, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends NeuroSci. (Pers. Ed.) 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. 2 plates. [DOI] [PubMed] [Google Scholar]
- 3.Herkenham M, Lynn AB, Johnson MR, Melvin LS, De CBR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in μ-opioid receptor patches of the rat caudate putamen nucleus. J. Neurosci. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parolaro D, Rubino T, Vigano D, Massi P, Guidali C, Realini N. Cellular mechanisms underlying the interaction between cannabinoid and opioid system. Curr. Drug Targets. 2010;11:393–405. doi: 10.2174/138945010790980367. [DOI] [PubMed] [Google Scholar]
- 6.Childers SR. Opioid receptor-coupled second messenger systems. Life Sci. 1991;48:1991–2003. doi: 10.1016/0024-3205(91)90154-4. [DOI] [PubMed] [Google Scholar]
- 7.Howlett AC. Pharmacology of cannabinoid receptors. Annu. Rev. Pharmacol. Toxicol. 1995;35:607–634. doi: 10.1146/annurev.pa.35.040195.003135. [DOI] [PubMed] [Google Scholar]
- 8.Childers SR, Fleming L, Konkoy C, Marckel DON, Pacheco M, Sexton T, Ward S. Opioid and Cannabinoid Receptor Inhibition of Adenylyl Cyclase in Braina. Ann. N. Y. Acad. Sci. 1992;654:33–51. doi: 10.1111/j.1749-6632.1992.tb25954.x. [DOI] [PubMed] [Google Scholar]
- 9.Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. J. Pharmacol. Exp. Ther. 1996;276:585–593. [PubMed] [Google Scholar]
- 10.Arvidsson U, Riedl M, Chakrabarti S, Lee J-H, Nakano AH, Dado RJ, Loh HH, Law P-Y, Wessendorf MW, Elde R. Distribution and targeting of a μ-opioid receptor (MOR1) in brain and spinal cord. J. Neurosci. 1995;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desroches J, Beaulieu P. Opioids and cannabinoids interactions: involvement in pain management. Curr. Drug Targets. 2010;11:462–473. doi: 10.2174/138945010790980303. [DOI] [PubMed] [Google Scholar]
- 12.Cichewicz DL, Martin ZL, Smith FL, Welch SP. Enhancement of μ opioid antinociception by oral Δ9-tetrahydrocannabinol: dose-response analysis and receptor identification. J. Pharmacol. Exp. Ther. 1999;289:859–867. [PubMed] [Google Scholar]
- 13.Reche I, Fuentes JA, Ruiz-Gayo M. Potentiation of Δ9-tetrahydrocannabinol-induced analgesia by morphine in mice: involvement of μ- and κ-opioid receptors. Eur. J. Pharmacol. 1996;318:11–16. doi: 10.1016/s0014-2999(96)00752-2. [DOI] [PubMed] [Google Scholar]
- 14.Cichewicz DL, Welch SP. Modulation of oral morphine antinociceptive tolerance and naloxone-precipitated withdrawal signs by oral Δ9-tetrahydrocannabinol. J. Pharmacol. Exp. Ther. 2003;305:812–817. doi: 10.1124/jpet.102.046870. [DOI] [PubMed] [Google Scholar]
- 15.Cichewicz DL, McCarthy EA. Antinociceptive synergy between Δ9-tetrahydrocannabinol and opioids after oral administration. J. Pharmacol. Exp. Ther. 2003;304:1010–1015. doi: 10.1124/jpet.102.045575. [DOI] [PubMed] [Google Scholar]
- 16.Williams J, Haller VL, Stevens DL, Welch SP. Decreased basal endogenous opioid levels in diabetic rodents: Effects on morphine and delta-9-tetrahydrocannabinoid-induced antinociception. Eur. J. Pharmacol. 2008;584:78–86. doi: 10.1016/j.ejphar.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 17.Roberts JD, Gennings C, Shih M. Synergistic affective analgesic interaction between delta-9-tetrahydrocannabinol and morphine. Eur. J. Pharmacol. 2006;530:54–58. doi: 10.1016/j.ejphar.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 18.Tham SM, Angus JA, Tudor EM, Wright CE. Synergistic and additive interactions of the cannabinoid agonist CP55,940 with μ opioid receptor and α2-adrenoceptor agonists in acute pain models in mice. Br. J. Pharmacol. 2005;144:875–884. doi: 10.1038/sj.bjp.0706045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trang T, Sutak M, Jhamandas K. Involvement of cannabinoid (CB1) receptors in the development and maintenance of opioid tolerance. Neuroscience (San Diego, CA, U. S.) 2007;146:1275–1288. doi: 10.1016/j.neuroscience.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 20.Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D, Ferrara P, Soubrie P, Breliere JC, Le Fur G. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 21.Mas-Nieto M, Pommier B, Tzavara ET, Caneparo A, Da NS, Le FG, Roques BP, Noble F. Reduction of opioid dependence by the CB1 antagonist SR141716A in mice: evaluation of the interest in pharmacotherapy of opioid addiction. Br. J. Pharmacol. 2001;132:1809–1816. doi: 10.1038/sj.bjp.0703990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarro M, Carrera MRA, Fratta W, Valverde O, Cossu G, Fattore L, Chowen JA, Gomez R, Del AI, Villanua MA, Maldonado R, Koob GF, Rodriguez d. F. F. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J. Neurosci. 2001;21:5344–5350. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cossu G, Ledent C, Fattore L, Imperato A, Bohme GA, Parmentier M, Fratta W. Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav. Brain Res. 2001;118:61–65. doi: 10.1016/s0166-4328(00)00311-9. [DOI] [PubMed] [Google Scholar]
- 24.Castane A, Robledo P, Matifas A, Kieffer BL, Maldonado R. Cannabinoid withdrawal syndrome is reduced in double mu and delta opioid receptor knockout mice. Eur. J. Neurosci. 2003;17:155–159. doi: 10.1046/j.1460-9568.2003.02409.x. [DOI] [PubMed] [Google Scholar]
- 25.Hojo M, Sudo Y, Ando Y, Minami K, Takada M, Matsubara T, Kanaide M, Taniyama K, Sumikawa K, Uezono Y. μ-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: electrophysiological and FRET assay analysis. J. Pharmacol. Sci. (Tokyo, Jpn.) 2008;108:308–319. doi: 10.1254/jphs.08244fp. [DOI] [PubMed] [Google Scholar]
- 26.Rios C, Gomes I, Devi LA. micro Opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br. J. Pharmacol. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pickel VM, Chan J, Kash TL, Rodriguez JJ, MacKie K. Compartment specific localization of cannabinoid 1 (CB1) and μ-opioid receptors in rat nucleus accumbens. Neuroscience (Oxford, U. K.) 2004;127:101–112. doi: 10.1016/j.neuroscience.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Salio C, Fischer J, Franzoni MF, Mackie K, Kaneko T, Conrath M. CB1-cannabinoid and μ-opioid receptor colocalization on postsynaptic target in the rat dorsal horn. Neuroreport. 2001;12:3689–3692. doi: 10.1097/00001756-200112040-00017. [DOI] [PubMed] [Google Scholar]
- 29.Lenard NR, Daniels DJ, Portoghese PS, Roerig SC. Absence of conditioned place preference or reinstatement with bivalent ligands containing mu-opioid receptor agonist and delta-opioid receptor antagonist pharmacophores. Eur. J. Pharmacol. 2007;566:75–82. doi: 10.1016/j.ejphar.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 30.Bhushan RG, Sharma SK, Xie Z, Daniels DJ, Portoghese PS. A bivalent ligand (KDN-21) reveals spinal δ and κ opioid receptors are organized as heterodimers that give rise to δ1 and κ2 phenotypes. selective targeting of δ-κ heterodimers. J. Med. Chem. 2004;47:2969–2972. doi: 10.1021/jm0342358. [DOI] [PubMed] [Google Scholar]
- 31.Portoghese PS, Larson DL, Sayre LM, Yim CB, Ronsisvalle G, Tam SW, Takemori AE. Opioid agonist and antagonist bivalent ligands. The relationship between spacer length and selectivity at multiple opioid receptors. J. Med. Chem. 1986;29:1855–1861. doi: 10.1021/jm00160a010. [DOI] [PubMed] [Google Scholar]
- 32.Portoghese PS, Larson DL, Yim CB, Sayre LM, Ronsisvalle G, Lipkowski AW, Takemori AE, Rice KC, Tam SW. Stereostructure-activity relationship of opioid agonist and antagonist bivalent ligands. Evidence for bridging between vicinal opioid receptors. J. Med. Chem. 1985;28:1140–1141. doi: 10.1021/jm00147a002. [DOI] [PubMed] [Google Scholar]
- 33.Daniels DJ, Kulkarni A, Xie Z, Bhushan RG, Portoghese PS. A Bivalent Ligand (KDAN-18) Containing delta-Antagonist and kappa-Agonist Pharmacophores Bridges delta2 and kappa1 Opioid Receptor Phenotypes. J. Med. Chem. 2005;48:1713–1716. doi: 10.1021/jm034234f. [DOI] [PubMed] [Google Scholar]
- 34.Akgün E, Javed MI, Lunzer MM, Smeester BA, Beitz AJ, Portoghese PS. Opioid mu agonist/mGluR5 antagonist bivalent ligands produce potent antinociception in mice with inflammatory pain. Proc. Natl. Acad. Sci. U.S.A. 2013 doi: 10.1073/pnas.1305461110. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harikumar KG, Akgun E, Portoghese PS, Miller LJ. Induced Association of micro Opioid (MOP) and Type 2 Cholecystokinin (CCK2) Receptors by Novel Bivalent Ligands. J. Med. Chem. 2010;53:2836–2842. doi: 10.1021/jm800174p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daniels DJ, Lenard NR, Etienne CL, Law P-Y, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc. Natl. Acad. Sci. U. S. A. 2005;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metzger TG, Paterlini MG, Ferguson DM, Portoghese PS. Investigation of the Selectivity of Oxymorphone- and Naltrexone-Derived Ligands via Site-Directed Mutagenesis of Opioid Receptors: Exploring the ‘Address’ Recognition Locus. J. Med. Chem. 2001;44:857–862. doi: 10.1021/jm000381r. [DOI] [PubMed] [Google Scholar]
- 38.Caille S, Parsons LH. Cannabinoid Modulation of Opiate Reinforcement through the Ventral Striatopallidal Pathway. Neuropsychopharmacology. 2006;31:804–813. doi: 10.1038/sj.npp.1300848. [DOI] [PubMed] [Google Scholar]
- 39.Azizi P, Haghparast A, Hassanpour-Ezatti M. Effects of CB1 receptor antagonist within the nucleus accumbens on the acquisition and expression of morphine-induced conditioned place preference in morphine-sensitized rats. Behav. Brain Res. 2009;197:119–124. doi: 10.1016/j.bbr.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Portoghese PS. Bivalent ligands and the message-address concept in the design of selective opioid receptor antagonists. Trends Pharmacol. Sci. 1989;10:230–235. doi: 10.1016/0165-6147(89)90267-8. [DOI] [PubMed] [Google Scholar]
- 41.Portoghese PS. From Models to Molecules: Opioid Receptor Dimers, Bivalent Ligands, and Selective Opioid Receptor Probes. J. Med. Chem. 2001;44:2259–2269. doi: 10.1021/jm010158+. [DOI] [PubMed] [Google Scholar]
- 42.Portoghese PS, Ronsisvalle G, Larson DL, Takemori AE. Synthesis and opioid antagonist potencies of naltrexamine bivalent ligands with conformationally restricted spacers. J. Med. Chem. 1986;29:1650–1653. doi: 10.1021/jm00159a014. [DOI] [PubMed] [Google Scholar]
- 43.Portoghese PS, Ronsisvalle G, Larson DL, Yim CB, Sayre LM, Takemori AE. Opioid agonist and antagonist bivalent ligands as receptor probes. Life Sci. 1982;31:1283–1286. doi: 10.1016/0024-3205(82)90362-9. [DOI] [PubMed] [Google Scholar]
- 44 (a).Francisco MEY, Seltzman HH, Gilliam AF, Mitchell RA, Rider SL, Pertwee RG, Stevenson LA, Thomas BF. Synthesis and Structure-Activity Relationships of Amide and Hydrazide Analogues of the Cannabinoid CB1 Receptor Antagonist N-(Piperidinyl)- 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR141716) J. Med. Chem. 2002;45:2708–2719. doi: 10.1021/jm010498v. [DOI] [PubMed] [Google Scholar]; (b) Lange JHM, Kruse CG. Medicinal chemistry strategies to CB1 cannabinoid receptor antagonists. Drug Discov. Today. 2005;10:693–702. doi: 10.1016/S1359-6446(05)03427-6. [DOI] [PubMed] [Google Scholar]; (c) Jagerovic N, Fernandez-Fernandez C, Goya P. CB1 cannabinoid antagonists: structure activity relationships and potential therapeutic applications. Curr. Top. Med. Chem. 2008;8:205–230. doi: 10.2174/156802608783498050. [DOI] [PubMed] [Google Scholar]
- 45.Seltzman HH, Carroll FI, Burgess JP, Wyrick CD, Burch DF. Tritiation of SR141716 by metalation-iodination-reduction: tritium-proton noe study. J. Labelled Compd. Radiopharm. 2002;45:59–70. [Google Scholar]
- 46.Daniels DJ. Thesis. University of Minnesota; Minneapolis, Minnesota: 2006. Bivalent Ligands as Probes for the Investigation of Opioid Receptor Dimerization. [Google Scholar]
- 47.Yekkirala AS, Lunzer MM, McCurdy CR, Powers MD, Kalyuzhny AE, Roerig SC, Portoghese PS. N-naphthoyl-beta-naltrexamine (NNTA), a highly selective and potent activator of mu/kappa-opioid heteromers. Proc. Natl. Acad. Sci. U. S. A. 2011;108:5098–5103. doi: 10.1073/pnas.1016277108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yekkirala AS. Thesis. University of Minnesota; Minneapolis, Minnesota: 2011. Elucidation the Role of Opioid Receptor Heteromers as Targets for Analgesic Drug Design. [Google Scholar]
- 49.Yekkirala AS, Kalyuzhny AE, Portoghese PS. An immunocytochemical-derived correlate for evaluating the bridging of heteromeric mu-delta opioid protomers by bivalent ligands. ACS Chem. Biol. 2013 doi: 10.1021/cb400113d. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng Y, Akgun E, Harikumar KG, Hopson J, Powers MD, Lunzer MM, Miller LJ, Portoghese PS. Induced Association of μ Opioid (MOP) and Type 2 Cholecystokinin (CCK2) Receptors by Novel Bivalent Ligands. J. Med. Chem. 2009;52:247–258. doi: 10.1021/jm800174p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Portoghese PS, Lunzer MM. Identity of the putative delta 1-opioid receptor as a delta-kappa heteromer in the mouse spinal cord. Eur. J. Pharmacol. 2003;467:233–234. doi: 10.1016/s0014-2999(03)01599-1. [DOI] [PubMed] [Google Scholar]
- 52.D’Amour FE, Smith DL. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941;72:74–79. [Google Scholar]
- 53.Dewey WL, Harris LS, Howes JF, Nuite JA. Effect of various neurohumoral modulators on the activity of morphine and the narcotic antagonists in the tail-flick and phenylquinone tests. J. Pharmacol. Exp. Ther. 1970;175:435–442. [PubMed] [Google Scholar]
- 54.Harris LS, Pierson AK. Narcotic antagonists in the benzomorphan series. J. Pharmacol. Exp. Ther. 1964;143:141–148. [PubMed] [Google Scholar]