The rapidly growing population of medically-underserved Chinese and other Asian Americans with low socioeconomic status and poor health status has not been adequately recognized and their health status is of increasing public health concern.1 The gaps in data and the lack of data disaggregation among Chinese and other Asian Americans mask dramatic health disparities and make it difficult to measure progress toward health disparities reduction initiatives.2,3 Medically-underserved Chinese Americans face multiple challenges in accessing health care due to poverty, limited English proficiency, lack of cultural orientation in care, and lack of culturally and linguistically proficient care. Almost seven in ten (69%) Chinese Americans are foreign-born compared with 13% of the US population and 8% of Whites,4 and 46% have limited English proficiency, compared with 9% of the US population and 6% of Whites.4 Chinese immigrants are significantly less likely to have a usual source of medical care (65%) than US-born Chinese (87%).5 Additionally, recent Chinese immigrants may be unfamiliar with the US health system and face challenges adjusting to that system. The lack of cultural concordance between patient and provider can lead to delays and treatment errors, and, ultimately, to poorer health outcomes.6–8
There is a paucity of national data on diabetes prevalence rates in Asian subpopulations. Diabetes prevalence rates for Asian Americans in the US range from 5% to 7.2% (unadjusted) and 7.3% (adjusted for age and sex) to 8.4% (adjusted for age), higher than rates for non-Hispanic Whites.9–11 The incidence of diabetes among Asian Americans doubled between 1997 and 2008, the highest increase among all racial/ethnic groups.11 McNeely and Boyko reported that while the age- and sex-adjusted prevalence was similar between Asian and White Americans, after adjusting for body mass index Asian Americans were noted to have a 60% higher adjusted prevalence of diabetes compared with Whites.9
Due to sampling issues, some recently reported diabetes prevalence rates among Chinese Americans may not be generalizable nor reflect the actual overall prevalence rates for Chinese Americans. For example, some of these studies conducted surveys only in English and excluded a substantial portion of Chinese and other Asian Americans with limited English proficiency, while others failed to adequately address the difficulty of recruiting hard-to-reach Chinese and other Asian immigrant populations and thus the surveys suffered from low response rates.12
Very few diabetes interventions have been developed and adapted for Chinese Americans or other Asian Americans.3,13 A few studies of Asian subgroups such as Chinese, Japanese, and Vietnamese in the United States, and Chinese in China and Australia, suggest that personal factors, such as family history, ethnicity, and language, can influence diabetes care and outcomes, and that culturally and linguistically appropriate treatment and health promotion programs can be effective in addressing these risk factors and improving outcomes.13–22 Research has shown that diabetes self-management education interventions designed specifically for underserved ethnic-specific groups (i.e., interventions that specifically incorporate socio-cultural aspects) can significantly improve outcomes including improvements in health behavior and knowledge, health status, and self-efficacy.23–26 Patient-provider language concordance has been associated with better medication adherence, fewer missed physician visits, provision of more health education, and patient perception of better care compared with language discordant pairs.27–29
Bodenheimer, et al.,30–32 have proposed systems-level changes using a “teamlet” model to extend the traditional 15-minute primary care visit. The teamlet model aims to improve patient care and patients’ self-management skills by expanding the visit to include one-on-one time with a trained health coach, who may be a medical assistant or other staff member, and by shifting routine tasks from the physician to the health coach, freeing up the physician to focus on his/her essential tasks.33 In the Bodenheimer model, the health coach has a pre-visit and post-visit meeting with the patient, assists the physician during the physician visit, and communicates with the patient between physician visits.33 This original teamlet approach did not specially address cultural and linguistic factors. A patient-centered model using a culturally and linguistically competent health coach may help address barriers to treatment for racial or ethnic minorities, who may have limited English proficiency, low health literacy, trouble advocating for themselves, or may face a variety of systems-level barriers. To date, this model has not been fully evaluated for its effectiveness in improving diabetes management (or other chronic diseases), nor has it been tailored for use among Chinese or other Asian Americans. A few studies have reported on interventions targeting Chinese Americans with diabetes, which display some of the features of the teamlet model. For example, a patient-centered diabetes education intervention tailored for Chinese Americans was successful in reducing HbA1c levels, systolic blood pressure, and body weight.14 These findings suggest that Bodenheimer’s teamlet model may be effective in improving diabetes treatment outcomes in Chinese Americans.
The purposes of this study were to adapt the Bodenheimer teamlet model for diabetes care among Chinese patients, to evaluate the adapted version for effectiveness in improving HbA1c levels in Chinese patients with diabetes over a six-month pilot intervention period, and to assess the feasibility of implementing this care model in a federally qualified health center setting.
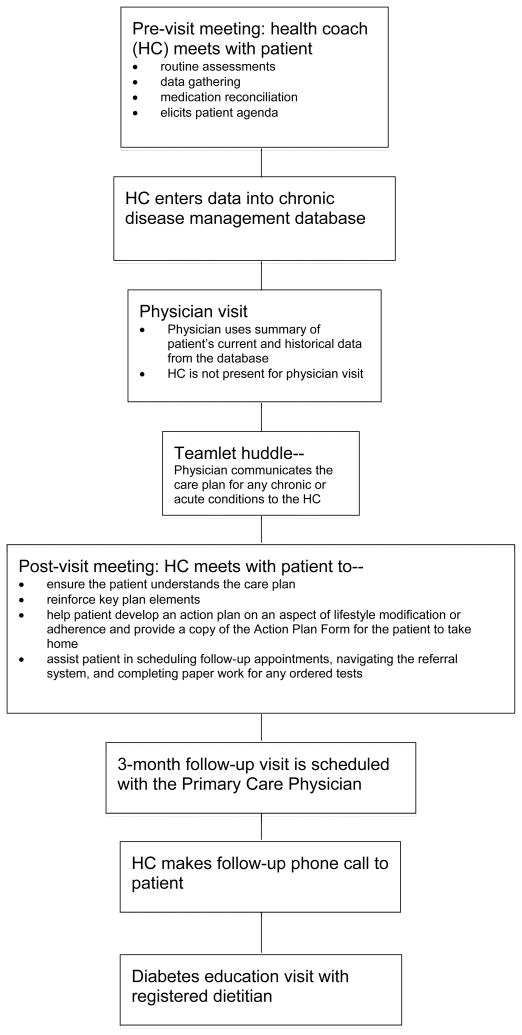
Methods
Intervention Design
A community-academic partnership between Asian Health Services (AHS), a safety net community health center in Oakland, California, the Association of Asian Pacific Community Health Organizations, and the University of California, Berkeley, Health Research for Action center, was initiated to address the rapidly growing Asian patient population with diabetes in the greater Oakland area served by AHS. The goal of this multi-disciplinary collaboration was to design, implement and evaluate community health strategies that could better serve this growing patient population. We developed, implemented, and evaluated an ethnic and language concordant teamlet model involving the use of a health coach specifically tailored for Chinese patients with diabetes enrolled at AHS (see Figure 1). The health coach role is based on the model proposed by Bodenheimer, et al.33 As part of this pilot intervention study, AHS clinic staff in the intervention unit implemented this model, referred to as the ‘AHS model’, with several modifications to the original Bodenheimer model: 1) Instead of staffing two health coaches for every one primary care physician, five health coaches worked with four physicians on the intervention unit; 2) the health coach was not present during the physician visit in order to preserve personnel resources; 3) after the physician visit, the physician communicated the care plan for any chronic or acute conditions to the health coach during a teamlet huddle; 4) nutritional counseling by a registered dietitian was added as a routine component of the intervention; and 5) all health coaches, the dietitian, and most physicians were ethnically and linguistically matched to their Chinese patients. Recommendations took into account cultural implications, e.g., a Chinese cultural diet. Physicians who did not speak their patient’s language would communicate with their patient through a clinic interpreter.
Figure 1.
AHS teamlet model
At AHS, the five health coaches were medical assistants (MAs) who underwent five one-hour health coach training sessions on workflow, diabetes, nutrition, medications, and communication techniques. The trainings consisted of didactic sessions, question and answer sessions, and role-playing. After the initial five-hour training, additional booster training sessions were provided as needed for the MAs during the year. During the intervention period for the study, there was no health coach turnover.
The primary responsibility of the health coach was to ensure that patients understood their diagnosis and treatment plan, knew how to manage their conditions, received adequate education, were able to navigate the health system, and had a liaison to help communicate concerns to their physician. The protocol required the health coach to meet with the patient just prior to the physician visit. During this pre-visit, the health coach would perform routine patient assessments (e.g., take vital signs), gather relevant data, conduct medication reconciliation, and elicit the patient’s agenda for talking with their physician. They also might provide language-appropriate diabetes education materials for the patient to take home to share with family members. The health coach would then enter the data into an electronic chronic disease management database, producing a summary report used by the physician during the physician-patient visit.
Immediately following the huddle, a post-visit would take place between the patient and health coach. Using communication techniques such as Ask-Tell-Ask and Closing the Loop1, 34 the health coach would ensure the patient understood the care plan and would reinforce key plan elements. Behavioral or self-care goals were then formulated collaboratively, captured on a Self-Management Action Plan Form, and copied for the patient to take home. Finally, the health coach would assist the patient in scheduling follow-up appointments, navigating the referral system, and making sure all necessary paper work was complete for any tests ordered by the physician as part of the care plan. The coach then scheduled a three-month follow-up visit scheduled with the primary care physician. As part of the pilot, patients in the intervention group were also scheduled for a face-to-face diabetes education visit (approximately 30–60 minutes) with a registered dietitian approximately two weeks after each physician visit. In addition, approximately one week after each physician visit, the health coach conducted a follow-up telephone call with the patient and/or family member to provide self-management support by reinforcing the care plan and action plan, answering basic questions, and serving as liaison to the physician to help address any patient concerns between clinic visits.
For the study, a complete intervention series would include at least three physician visits, three dietitian visits, and three follow-up phone calls from the health coach over a period of approximately six months. Patients enrolled in the intervention were included in the analysis regardless of the actual number of visits under an intention-to-treat approach. Control patients had no specified number of provider visits during the same time period (“usual care”). The mean number of chronic care physician visits during the six months following the baseline physician visit did not differ significantly by group (averaging 2.5 visits for both intervention and control groups).
Study Design
Study participants were recruited from two outpatient medical care units at AHS, a federally qualified health center that primarily serves low-income Asian Americans, many of whom are immigrants. Patients with a diagnosis of type 2 diabetes who had an upcoming chronic care physician visit were identified from each unit. One unit implemented the teamlet model (intervention unit) while the other unit offered usual care (control unit). Study participants were not randomly assigned to intervention and control groups, but instead were conveniently assigned to control and intervention groups based on the medical unit where they normally received their care. The two units participating in the pilot have comparable types of patients. The intervention unit is staffed by a mix of internists and family physicians, while the control unit has only family physicians. Clinic staff members screened potential participants by chart review. Inclusion criteria included being age 18 years or older, a current clinic patient, Cantonese-, Mandarin-, or English-speaking, of Chinese heritage, and having an ICD-9-CM diagnosis of type 2 diabetes. Exclusion criteria included significantly impaired cognition or Alzheimer’s disease, schizophrenia, HbA1c levels < 6% or > 11%, and recent history (last six months) of cancer (except certain types of unmetastasized skin cancer), myocardial infarction, cardiac surgery, or stroke. Eligible patients were invited to join the study, and, if interested, scheduled for an in-person interview appointment with a study research assistant.
At the intake appointment, the research assistant obtained informed consent for the study and conducted a baseline interview in Cantonese, Mandarin, or English, depending on the patient’s preferred language. Participants recruited from the control unit received usual care for their diabetes, while participants recruited from the intervention unit began receiving care for their diabetes under the teamlet model, beginning with the patient’s next chronic care physician visit (baseline physician visit). The research assistants also abstracted relevant information from medical records around the time of the baseline interview and approximately three and six months following the baseline physician visit. Follow-up study interviews were conducted about three and six months after the baseline interview and were conducted either in-person or over the phone. Of the 142 potential participants who were screened and found to be eligible for the study, 97 enrolled in the study, giving a participation rate of 68%. Two participants were dropped from the study after the baseline interview because they no longer met eligibility requirements such as being a current AHS patient. Three participants did not have a follow-up HbA1c after their baseline assessment, giving a total of 92 participants (n=46 intervention participants and n=46 control participants) for analysis of the primary endpoint measure, 6-month HbA1c level. Data were collected between May 2009 and June 2010. The institutional review board at the University of California, Berkeley, approved all study protocols and instruments.
Chinese Community Research Advisory Committee (CCRAC)
An advisory committee was formed to advise the research team on the study design, implementation of the intervention, and interpretation and dissemination of the results. Members of this committee included AHS patients with diabetes and their family members, as well as professionals from the community with expertise in diabetes and/or experience working with the Asian American community. The CCRAC met regularly during the study.
Interview
Topics covered in the study interview included demographic questions (baseline visit only), time since diabetes diagnosis (baseline only), self-rated health status, adequacy of financial resources, language spoken at home, and employment status.
Medical record abstraction
Data abstracted from the patient’s medical record included type of health insurance (if any), height, weight, dates and values of selected clinical tests including HbA1c level, and measures of systolic and diastolic blood pressure. The baseline abstraction included the most recent HbA1c value from the six months prior to the baseline physician visit (‘baseline HbA1c’, the value used to screen for inclusion in the study). Follow-up abstractions recorded the results of clinical tests performed closest to the three- and six- month time points after the baseline physician visit.
Data analysis
Analysis of the data was conducted on two samples – a larger sample comprising 92 participants with a follow-up HbA1c test performed at any time during the study (range 4.1 – 11.2 months, mean 6.9 months), and a smaller sample of 78 participants with a follow-up HbA1c test performed during a tighter interval of 5 to 8.5 months after entering the study. In addition, the larger sample includes two participants whose baseline HbA1c levels were slightly outside the initial recruitment inclusion range of 6–11%.
We conducted comparisons of baseline frequencies and means of demographic characteristics between control and intervention groups using the Fisher’s exact test (categorical variables) or Student‘s t-test (continuous variables). Unadjusted analyses of the change in the primary outcome measure, HbA1c level, consisted of comparison of the mean change between the intervention and control participants, using Student‘s t-tests. In addition, we categorized HbA1c levels as ‘Controlled’ (<7%) or ‘Not well-controlled’ (≥7%)35 and compared proportions in each category by group at baseline and follow-up, using Fisher’s exact test. These analyses were conducted using SAS statistical software (v9.2, SAS Institute, Cary, NC).
For adjusted analyses, control participants were matched to participants in the intervention group according to a propensity score. For the smaller data set (n=78), the propensity score was based on age, years since diagnosis, health insurance status (private, public, or no insurance), and sex. For the larger data set (n=92), the propensity score was based on age, years since diagnosis, health insurance status (private, public, or no insurance), sex, and number of months between entering the study and HbA1c follow-up test. We produced histograms demonstrating the distribution of estimated propensity scores by intervention vs. control group with both the larger and smaller data sets to test the validity of estimating the adjusted mean among the intervention and control participants. Propensity score matching analyses were conducted using the psmatch2 command in Stata 10 for Windows (StataCorp, 2007), using the nonparametric bootstrapping option to derive statistical inference.
Results
Demographics
We compared demographic characteristics of control and intervention participants in both the smaller (n=78) and larger (n=92) samples, and found no statistically significant differences (see Table 1 for details).
Table 1.
Characteristics of intervention and control group participants at baseline
| Smaller sample* (n=78) | Larger sample** (n=92) | |||
|---|---|---|---|---|
| Control | Intervention | Control | Intervention | |
| n=39 | n=39 | n=46 | n=46 | |
|
|
||||
| Sex, n (%) | ||||
| Male | 11 (28.2) | 16 (41.0) | 14 (30.4) | 18 (39.1) |
| Female | 28 (71.8) | 23 (60.0) | 32 (69.6) | 28 (60.9) |
| Age, mean (SD) | 66.8 (11.6) | 66.5 (9.8) | ||
| Primary language spoken at home, n (%) | ||||
| Cantonese | 31 (79.5) | 24 (61.5) | 37 (80.4) | 30 (65.2) |
| Mandarin | 3 (7.7) | 9 (23.1) | 3 (6.5) | 9 (19.6) |
| English | 0 (0) | 0 (0) | 0 (0) | 1 (2.2) |
| Other | 4 (10.3) | 4 (10.3) | 5 (10.9) | 4 (8.7) |
| More than one language | 1 (2.6) | 2 (5.1) | 1 (2.2) | 2 (4.4) |
| Years living in US, mean (SD) | 17.8 (8.2) | 15.2 (9.6) | 18.2 (8.3) | 16.6 (12.5) |
| Marital status, n (%) | ||||
| Married or living with partner | 29 (74.4) | 30 (76.9) | 34 (73.9) | 35 (76.1) |
| Divorced, separated, or widowed | 9 (23.1) | 8 (20.5) | 11 (23.9) | 10 (21.7) |
| Never married | 1 (2.6) | 1 (2.6) | 1 (2.2) | 1 (2.2) |
| Education, n (%) | ||||
| <High school | 30 (76.9) | 24 (61.5) | 32 (69.6) | 30 (65.2) |
| High school graduate | 4 (10.3) | 9 (23.1) | 6 (13.0) | 9 (19.6) |
| Some college or college graduate | 5 (12.8) | 6 (15.4) | 8 (17.4) | 7 (15.2) |
| Health insurance status, n (%) | ||||
| Private insurance | 0 (0) | 1 (2.6) | 0 (0) | 3 (6.5) |
| Public insurance | 29 (74.4) | 26 (66.7) | 34 (73.9) | 30 (65.2) |
| Uninsured | 10 (25.6) | 12 (30.8) | 12 (26.1) | 13 (28.3) |
| Health status, n (%)*** | ||||
| Poor | 9 (23.7) | 11 (29.0) | 10 (22.7) | 11 (24.4) |
| Fair | 21 (55.3) | 23 (60.5) | 26 (59.1) | 29 (64.4) |
| Good | 5 (13.2) | 4 (10.5) | 5 (11.4) | 4 (8.9) |
| Very good | 3 (7.9) | 0 (0) | 3 (6.8) | 1 (2.2) |
| Excellent | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Participants with follow-up HbA1c between 5 and 8.5 months after baseline MD visit
All participants with follow-up HbA1c (regardless of date of follow-up)
Sum of responses is less than total number of participants due to missing responses for some participants.
NB: No statistically significant differences were found between control and intervention groups in any factor at a level of p<.05, regardless of which sample was analyzed.
Mean change in HbA1c
Unadjusted analyses
The mean baseline HbA1c was similar among participants in the intervention group (7.59%) and in the control group (7.62%) for the smaller sample (n=78) (Table 2). At follow-up, between 5 and 8.5 months after the initial physician visit during the study period, mean HbA1c had decreased by .40% (95% CI= −0.65, −0.15) to 7.19% among intervention participants, while mean HbA1c among control participants increased slightly (+.04%, 95% CI= −0.41, 0.49) to 7.65%. This difference between groups in the mean change in HbA1c level did not reach statistical significance (p=.1045). See Table 2 for results of analysis with the larger sample (n=92).
Table 2.
Comparison of change in HbA1c in intervention and control groups between baseline and follow-up (unadjusted)
| HbA1c | Smaller sample* (n=78) | Larger sample** (n=92) | ||||
|---|---|---|---|---|---|---|
| Control | Intervention | p value*** | Control | Intervention | p value*** | |
| n=39 mean (SD) | n=39 mean (SD) | n=46 mean (SD) | n=46 mean (SD) | |||
|
|
||||||
| Mean Baseline | 7.62 (.75) | 7.59 (.81) | 7.62 (.75) | 7.60 (1.16) | ||
| Mean 6 Months | 7.65 (1.33) | 7.19 (.96) | 7.63 (1.23) | 7.24 (1.02) | ||
| Mean of Difference (6 Mos - Baseline) | .04 (1.44) | −0.40 (0.79) | 0.1045 | 0.02 (1.36) | −0.36 (1.09) | 0.1423 |
| n (%) | n (%) | n (%) | n (%) | |||
|
|
||||||
| Well-Controlled (<7%) (Baseline) | 7 (18.0) | 10 (25.6) | 0.5843 | 9 (19.6) | 13 (28.3) | 0.4640 |
| Well-Controlled (<7%) (6 Mos) | 11 (28.2) | 18 (46.2) | 0.1593 | 11 (23.9) | 21 (45.7) | 0.0480 |
Participants with follow-up HbA1c between 5 and 8.5 months after baseline MD visit
All participants with follow-up HbA1c (regardless of date of follow-up)
p value based on T-test for continuous variables and Fisher’s exact test for categorical variables
Adjusted analyses
When we ran the propensity score matched analyses, the mean difference in HbA1c between baseline and follow-up measures (between 5 and 8.5 months after the initial physician visit) was −.40% among intervention participants and +.03% among control participants for the smaller sample (n=78). The difference between these means was not statistically significant (p=.295). When we re-ran the analysis including all participants with a follow-up HbA1c regardless of time since baseline physician visit (n=92), intervention participants showed a decrease in HbA1c of −.36, while control participants demonstrated an increase of .32. This difference between group mean change in HbA1c over time demonstrated a trend in the direction hypothesized (p=.064). The histograms showing the distribution of estimated propensity scores for the intervention and control groups using both the smaller and larger data sets indicated sufficient overlap between both groups, so that nearly all observations can be used to derive the adjusted HbA1c mean estimate (data not shown). This finding provides support for estimating the adjusted mean among the intervention and control participants.
Categorical HbA1c
At baseline, 18.0% (95% CI= 8.7, 33.0) of control group participants and 25.6% (95% CI= 14.4, 41.2) of intervention group participants had HbA1c results that were considered ‘well-controlled’ (p=.5843) in the smaller sample (Table 2). At follow-up, both groups had an increase in the proportion of participants with well-controlled HbA1c levels, with a larger increase in the intervention group (increased to 46.2% (95% CI= 31.6, 61.4)) compared to the control group (increased to 28.2% (95% CI= 16.4, 43.9)), although this difference between groups was not significant (p=.1593). However when we ran the analysis with the larger sample (including any participant with a follow-up HbA1c regardless of timing of test), we noted a statistically significant difference between the intervention group value of 45.7% (95% CI= 32.2, 59.8) and the control group value of 23.9% (95% CI= 13.8, 38.1), p=.0480 (see Table 2 for details).
Discussion
This paper describes the implementation and evaluation of a primary care culturally-tailored, linguistically appropriate health coach intervention, based on the Bodenheimer teamlet model, among Chinese American patients at a community clinic who had type 2 diabetes. We assessed the feasibility of this approach for full clinical implementation. Our primary outcome was HbA1c level over a 6-month period. While our results were not statistically significant, we did see a decrease in % HbA1c in the intervention group at 6-month follow-up, compared to a slight increase in % HbA1c in control participants. The direction and magnitude of this change is comparable to that seen in other intervention studies (0.4 – 1% change in HbA1c).14,15,36–38 We believe the primary reason this difference between groups was not significant is the lack of adequate statistical power, given the small sample size in the pilot.
The clinic staff anecdotally noted several possible mechanisms for decreased HbA1c levels among patients who received health coaching. Health coaches were able to catch errors in medication (e.g., a patient not taking medications or taking the wrong dosage), and to correct such errors early, before the patient’s next physician visit about 3 months later. Health coaches also helped patients navigate the medical system, which increased adherence to physician recommendations. For example, a health coach assisted a patient in obtaining his medications through his health insurance. The intervention may also have prevented relapses in diet and exercise regimen adherence by providing reminders and encouragement. Health coaching, especially through the phone calls made between clinic visits, provided increased support for patients to manage their diabetes.
This study demonstrated that it is feasible to implement an adapted version of Bodenheimer’s teamlet model in a community clinic with a large, medically-underserved Asian American patient population. We were able to translate the intervention to fit the resources available at the clinic and to tailor it for a specific ethnic group, Chinese Americans. The intervention was successfully and enthusiastically implemented in the unit that was selected for the intervention pilot. The intervention team maintained active interest throughout. AHS has since expanded the use of the culturally and linguistically concordant teamlet model with some modifications in order to include patients with other chronic care conditions and other units within the clinic.
While AHS hopes to implement this health coach model in all of their medical units, it faces significant challenges. The cost of sustaining the model (primarily the cost of additional MAs) is one challenge. Enabling services, under which health coaching may fall, are largely unreimbursed by health insurance plans. Studies such as ours are invaluable in demonstrating the value of the health coach model and supporting current advocacy efforts for payment reform (e.g., the Patient-Centered Medical Home).39,40 Eighty-five percent of community health centers report that primary care visits for patients with limited English proficiency take longer than visits with an English proficient patient requiring similar care, ranging from an additional 5 to 30 minutes per visit.41 Minority groups who are foreign-born and have limited English proficiency have greater language and cultural barriers to receiving care. Managing comorbidities and complex medical conditions often requires enabling services such as interpretation, navigation services, or health coaching.39 Despite these challenges, AHS and community health centers nationally have accepted the idea of leveraging use of non-physician workers to improve chronic disease care as these services are critical for their medically underserved patients’ access to care and help to prevent emergency department visits.42
Another challenge is changing traditional ideas of what falls under the domain of MAs’ duties; although no formal process evaluation was conducted with the study’s coaches, informal feedback provided by the MAs at monthly Practice Improvement Team meetings (which ensured buy-in from physicians, MAs, and the unit clerk) indicated that some MAs were reluctant to take on the additional duties required of health coaches. On the other hand, many MAs were excited by the opportunity to become more involved in patient care and to have a greater impact on care. The incorporation of a combination of financial incentives and Practice Improvement Team meetings helped to overcome reluctance on the part of some MAs. Allowing the unit staff to see how the teamlet model was leading to improved health outcomes helped to sustain their motivation. Feedback from patients has led to booster sessions for the health coaches and new resources for patients, such as the development of new diabetes health education materials for patients at a more appropriate literacy level and for family members of patients with diabetes on how to support their relative with diabetes. Organizational buy-in from management, physicians, and front-line staff is key, but requires significant organizational and cultural change.
Strengths
The pilot site for the intervention was an ideal community partner given existing interest in the health coach model, past experience with research and evaluation, and the ability to easily identify workers who could be trained to deliver care to predominantly Cantonese- or Mandarin-speaking patients. The site had a diabetes registry already in place, which facilitated identification of patients to recruit for the study. A community advisory committee was maintained throughout the process to ensure the research included the views of patients with diabetes, family members of those with diabetes, and community members. The university-based research team held regular meetings or conference calls with the clinic-based research team, sharing in troubleshooting as the intervention study rolled out.
Another key component was the active engagement of a physician leader and a registered dietitian at the clinical site who gave professional guidance during the project and made organizational assessments of how the rollout was progressing (e.g., the training of health coaches and follow-up of participants). The dietitian also developed a nutrition training curriculum for the health coaches, which included in-person training sessions and observations of the dietitian by the health coaches during dietician counseling sessions with patients with diabetes.
Limitations
With only one site, we knew workers in other units were likely aware of the intervention, and the number of Chinese patients with diabetes eligible for recruitment was small. In fact, the threat of inadequate enrollment of participants was a concern throughout the project. For example, we made mid-course corrections to enroll two additional patients in an attempt to maintain adequate power to detect a difference in our primary outcome measure. Even with this constant attention to sample size, we fell short of recruitment goals. Conducting research among medically-underserved, immigrant communities may present significant challenges such as the lack of familiarity and experience with participating in research, language barriers, lack of transportation and time to participate in follow-up visits, and health insurance restricting follow-up medical visits.43–46 The resulting lack of adequate sample size informed the choice of analysis methodology. In particular, instead of using traditional multivariate analytical procedures, we attempted to maximize statistical power by matching on propensity score based on possible confounders.
Another unanticipated issue was that, with a limited study budget, we were dependent on a physician ordering the HbA1c test and on the patient adhering to recommended intervals for testing and interviewing. This resulted in a wider range of timing for measurement of HbA1c levels. We limited our main analyses to participants with a “six-month” measurement between 5 and 8.5 months following the baseline physician visit, thus reducing our effective sample size from 92 to 78. While this parallels the physician’s real world experience of caring for patients, it makes analysis more difficult since each patient could be seen as having a slightly different dose of the intervention (e.g., is five months of the intervention less effective than seven months?). Our second propensity score matched analysis includes the full group of study participants, regardless of date of determination of the follow-up HbA1c value, and attempts to adjust for this variation in ‘dose’ by including the time interval between entering the study and measurement of the follow-up HbA1c as an additional matching criterion.
Another limitation of the study was that participants were not randomly assigned to intervention and control groups. They were conveniently assigned to intervention and control groups based on the medical unit where they normally received their care. While randomization might be preferable in a full-scale clinical trial, we decided to proceed without randomization for several reasons. The health coaching was being implemented in only one of the units for logistical and cost considerations, as well as to prevent contamination of control participants that might occur if all participants were seen by the same staff. Randomly assigning patients to a different physician for the purpose of the pilot study would also disrupt the ongoing primary care relationship. There may be differences between these units for which we cannot control, e.g., the number and types of health care providers in each unit. However, we note that our intervention and control participants are comparable in demographic and health characteristics at baseline (Table 1).
Conclusions
This pilot study suggests health coaches may improve clinical outcomes for a medically-underserved population. While not all our findings are statistically significant, they suggest that the use of a culturally-tailored, linguistically appropriate teamlet model of care for Chinese Americans with type 2 diabetes is associated with a meaningful decrease in HbA1c (e.g., comparable in magnitude to the addition of a diabetes medication). We feel there is sufficient success in the study to warrant a larger trial with multiple sites over a longer period of time, which would have adequate power to detect an intervention-related difference in HbA1c levels, allow for examination of equally important clinical endpoints (e.g., blood pressure), and overcome the current study’s limitation of small sample size. The teamlet model may also be a promising component for the Patient-Centered Medical Home.
Footnotes
Ask-Tell-Ask is a patient-directed motivational interviewing technique to provide needed information to the patient and check for understanding of the information. Closing the Loop is a related technique to assess patient’s understanding of new information by asking the patient to restate his or her understanding of the information.
Contributor Information
Susan L. Ivey, Email: sivey@berkeley.edu, Health Research for Action, School of Public Health, University of California, Berkeley, 2140 Shattuck Avenue, 10th Floor, Berkeley, CA 94704-7388; phone: 510.643.1883; fax: 510.643.7679.
Winston Tseng, Email: winston@berkeley.edu, Health Research for Action, School of Public Health, University of California, Berkeley, 2140 Shattuck Avenue, 10th Floor, Berkeley, CA 94704-7388; phone: 510.643.4461; fax: 510.643.7679.
Elaine Kurtovich, Email: elainek@berkeley.edu, Health Research for Action, School of Public Health, University of California, Berkeley, 2140 Shattuck Avenue, 10th Floor, Berkeley, CA 94704-7388; phone: 510.643.7456; fax: 510.643.7679.
Ben Lui, Email: blui@ahschc.org, Asian Medical Center site director, Asian Health Services, 818 Webster Street, Oakland, CA 94607-4220; phone: 510.986.6800 x461.
Rosy Chang Weir, Email: rcweir@aapcho.org, Association of Asian Pacific Community Health Organizations, 300 Frank Ogawa Plaza, Suite 620, Oakland, CA 94612; phone: 510.272.9536 x107; fax: 510.272.0817.
Jing Liu, Email: jliu@ahschc.org, Asian Health Services, 818 Webster Street, Oakland, CA 94607-4220; phone: 510.986.6830 x287.
Hui Song, Email: hsong@aapcho.org, Association of Asian Pacific Community Health Organizations, 300 Frank Ogawa Plaza, Suite 620, Oakland, CA 94612; phone: 510.272.9536 x119; fax: 510.272.0817.
May Wang, Email: maywang@ucla.edu, Department of Community Health Sciences, UCLA School of Public Health, P.O. Box 951772, Los Angeles, CA 90095-1772; phone: 310.206.5306; fax: 310.794.1805.
Alan Hubbard, Email: hubbard@stat.berkeley.edu, School of Public Health, University of California, Berkeley, 101 Haviland Hall, MC 7358, Berkeley, CA 94720; phone: 510.643.6160; fax: 510.643.5163.
References
- 1.Weir R, Tseng W, Yen IH, Caballero J. Primary health-care delivery gaps among medically underserved Asian American and Pacific Islander populations. Public Health Reports. 2009;124(6):831–840. doi: 10.1177/003335490912400611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghosh C. Healthy People 2010 and Asian Americans/Pacific Islanders: Defining a baseline of information. Am Public Health Assoc. 2003;93:2093–2098. doi: 10.2105/ajph.93.12.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh C. A national health agenda for Asian Americans and Pacific Islanders. JAMA: The Journal of the American Medical Association. 2010;304(12):1381–1382. doi: 10.1001/jama.2010.1358. [DOI] [PubMed] [Google Scholar]
- 4.American Community Survey. Washington, D.C: U.S. Census Bureau; 2007–2009. [Google Scholar]
- 5.Jang M, Lee E, Woo K. Income, language, and citizenship status: Factors affecting the health care access and utilization of Chinese Americans. Health and Social Work. 1998;23(2) doi: 10.1093/hsw/23.2.136. [DOI] [PubMed] [Google Scholar]
- 6.Frisbie W, Cho Y, Hummer R. Immigration and the health of Asian and Pacific Islander adults in the United States. American Journal of Epidemiology. 2001;153(4):372. doi: 10.1093/aje/153.4.372. [DOI] [PubMed] [Google Scholar]
- 7.Ngo Metzger Q, Massagli MP, Clarridge BR, Manocchia M, Davis RB, Iezzoni LI, Phillips RS. Linguistic and cultural barriers to care. Journal of General Internal Medicine. 2003;18(1):44–52. doi: 10.1046/j.1525-1497.2003.20205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma GX. Between two worlds: the use of traditional and Western health services by Chinese immigrants. J Community Health. 1999;24(6):421–437. doi: 10.1023/a:1018742505785. [DOI] [PubMed] [Google Scholar]
- 9.McNeely MJ, Boyko EJ. Type 2 diabetes prevalence in Asian Americans. Diabetes Care. 2004;27(1):66–69. doi: 10.2337/diacare.27.1.66. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 11.Lee JWR, Brancati FL, Yeh HC. Trends in the prevalence of type 2 diabetes in Asians versus whites. Diabetes Care. 2011;34(2):353. doi: 10.2337/dc10-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Islam NS, Khan S, Kwon S, Jang D, Ro M, Trinh-Shevrin C. Methodological issues in the collection, analysis, and reporting of granular data in Asian American populations: Historical challenges and potential solutions. Journal of Health Care for the Poor and Underserved. 2010;21(4):1354. doi: 10.1353/hpu.2010.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawthorne K, Robles Y, Cannings-John R, Edwards A. Culturally appropriate health education for type 2 diabetes in ethnic minority groups: A systematic and narrative review of randomized controlled trials. Diabetic Medicine. 2010;27(6):613–623. doi: 10.1111/j.1464-5491.2010.02954.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang C, Chan S. Culturally tailored diabetes education program for Chinese Americans: A pilot study. Nursing Research. 2005;54(5):347–353. doi: 10.1097/00006199-200509000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Sun J, Wang Y, Chen X, Chen Y, Feng Y, Zhang X, Pan Y, Hu T, Xu J, Du L. An integrated intervention program to control diabetes in overweight Chinese women and men with type 2 diabetes. Asia Pacific Journal of Clinical Nutrition. 2008;17(3):514–524. [PubMed] [Google Scholar]
- 16.Fisher L, Skaff MM, Chesla CA, Chun KM, Mullan JT, Kanter RA, Gardiner PS. Disease management advice provided to African-American and Chinese-American patients with type 2 diabetes. Diabetes Care. 2004;27(9):2249–2250. doi: 10.2337/diacare.27.9.2249. [DOI] [PubMed] [Google Scholar]
- 17.Liao D, Asberry P, Shofer J, Callahan H, Matthys C, Boyko E, Leonetti D, Kahn S, Austin M, Newell L. Improvement of BMI, body composition, and body fat distribution with lifestyle modification in Japanese Americans with impaired glucose tolerance. Diabetes Care. 2002;25(9):1504–1510. doi: 10.2337/diacare.25.9.1504. [DOI] [PubMed] [Google Scholar]
- 18.Carr D, Utzschneider K, Boyko E, Asberry P, Hull R, Kodama K, Callahan H, Matthys C, Leonetti D, Schwartz R. A reduced-fat diet and aerobic exercise in Japanese Americans with impaired glucose tolerance decreases intra-abdominal fat and improves insulin sensitivity but not β-cell function. Diabetes. 2005;54(2):340–347. doi: 10.2337/diabetes.54.2.340. [DOI] [PubMed] [Google Scholar]
- 19.Pham T. Samuels & Associates, ed. , editor. The social & environmental experience of diabetes: Implications for diabetes prevention, management and treatment programs: A series of case studies. Woodland Hills, CA: The California Endowment; 2004. Asian and Pacific Islander case study: The diabetic Vietnamese population of San Diego County. [Google Scholar]
- 20.Fu D, Fu H, McGowan P, Shen Y, Zhu L, Yang H, Mao J, Zhu S, Ding Y, Wei Z. Implementation and quantitative evaluation of chronic disease self-management programme in Shanghai, China: Randomized controlled trial. Bulletin of the World Health Organization. 2003;81:174–182. [PMC free article] [PubMed] [Google Scholar]
- 21.Swerissen H, Belfrage J, Weeks A, Jordan L, Walker C, Furler J, McAvoy B, Carter M, Peterson C. A randomised control trial of a self-management program for people with a chronic illness from Vietnamese, Chinese, Italian and Greek backgrounds. Patient Education and Counseling. 2006;64(1–3):360–368. doi: 10.1016/j.pec.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Census Bureau. The Asian Population: 2000. Washington, DC: U.S. Census Bureau; 2002. [Google Scholar]
- 23.Kehoe K, Melkus G, Newlin K. Culture within the context of care: An integrative review. Ethnicity & Disease. 2003;13(3):344–353. [PubMed] [Google Scholar]
- 24.Eakin E, Bull S, Glasgow R, Mason M. Reaching those most in need: A review of diabetes self-management interventions in disadvantaged populations. Diabetes/Metabolism Research and Reviews. 2002;18(1):26–35. doi: 10.1002/dmrr.266. [DOI] [PubMed] [Google Scholar]
- 25.Philis-Tsimikas A, Walker C. Improved care for diabetes in underserved populations. Journal of Ambulatory Care Management. 2001;24(1):39–43. doi: 10.1097/00004479-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Gilmer TP, Philis-Tsimikas A, Walker C. Outcomes of Project Dulce: A culturally specific diabetes management program. The Annals of Pharmacotherapy. 2005;39(5):817–822. doi: 10.1345/aph.1E583. [DOI] [PubMed] [Google Scholar]
- 27.Ngo-Metzger Q, Sorkin DH, Phillips RS, Greenfield S, Massagli MP, Clarridge B, Kaplan SH. Providing high-quality care for limited English proficient patients: the importance of language concordance and interpreter use. Journal of General Internal Medicine. 2007;22:324–330. doi: 10.1007/s11606-007-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manson A. Language concordance as a determinant of patient compliance and emergency room use in patients with asthma. Medical Care. 1988:1119–1128. doi: 10.1097/00005650-198812000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Traylor AH, Schmittdiel JA, Uratsu CS, Mangione CM, Subramanian U. Adherence to Cardiovascular Disease Medications: Does Patient-Provider Race/Ethnicity and Language Concordance Matter? Journal of General Internal Medicine. 2010;25(11):1172–1177. doi: 10.1007/s11606-010-1424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodenheimer T. Helping patients improve their health-related behaviors: What system changes do we need? Disease Management. 2005;8(5):319–330. doi: 10.1089/dis.2005.8.319. [DOI] [PubMed] [Google Scholar]
- 31.Bodenheimer T. Planned visits to help patients self-manage chronic conditions. American Family Physician. 2005;72(8):1454, 1456. [PubMed] [Google Scholar]
- 32.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA: The Journal of the American Medical Association. 2002;288(19):2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 33.Bodenheimer T, Laing B. The teamlet model of primary care. The Annals of Family Medicine. 2007;5(5):457–461. doi: 10.1370/afm.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bodenheimer T, Grumbach K. Improving primary care: Strategies and tools for a better practice. New York: McGraw-Hill Medical; 2006. [Google Scholar]
- 35.Executive summary: Standards of medical care in diabetes--2010. Diabetes Care. 2010;33(Supplement 1):S4–S10. doi: 10.2337/dc10-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker EA, Shmukler C, Ullman R, Blanco E, Scollan-Koliopoulus M, Cohen HW. Results of a successful telephonic intervention to improve diabetes control in urban adults: A randomized trial. Diabetes Care. 2011;34(1):2–7. doi: 10.2337/dc10-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee K, Palacio C, Alexandraki I, Stewart E, Mooradian AD. Increasing access to health care providers through medical home model may abolish racial disparity in diabetes care: Evidence from a cross-sectional study. Journal of the National Medical Association. 2011;103(3):251–256. doi: 10.1016/s0027-9684(15)30293-5. [DOI] [PubMed] [Google Scholar]
- 38.Gary TL, Batts-Turner M, Yeh HC, Hill-Briggs F, Bone LR, Wang NY, Levine DM, Powe NR, Saudek CD, Hill MN. The effects of a nurse case manager and a community health worker team on diabetic control, emergency department visits, and hospitalizations among urban African Americans with type 2 diabetes mellitus: A randomized controlled trial. Archives of Internal Medicine. 2009;169(19):1788–1794. doi: 10.1001/archinternmed.2009.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Association of Asian Pacific Community Health Organizations. The Role of Enabling Services in Patient-Centered Medical Homes. 2010. [Google Scholar]
- 40.NCQA’s Patient-Centered Medical Home (PCMH) 2011. National Committee for Quality Assurance; 2011. [Google Scholar]
- 41.National Association of Community Health Centers. Serving Patients with Limited English Proficiency: Results of a Community Health Center Survey. 2008. [Google Scholar]
- 42.Association of Asian Pacific Community Health Organizations, National Association of Community Health Centers. Highlighting the Role of Enabling Services at Community Health Centers: Collecting Data to Support Service Expansion & Enhanced Funding. 2010. [Google Scholar]
- 43.Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, Tilburt J, Baffi C, Tanpitukpongse TP, Wilson RF. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 44.Robinson JM, Trochim WMK. An examination of community members’, researchers’ and health professionals’ perceptions of barriers to minority participation in medical research: An application of concept mapping. Ethn Health. 2007;12(5):521–539. doi: 10.1080/13557850701616987. [DOI] [PubMed] [Google Scholar]
- 45.Keyzer JF, Melnikow J, Kuppermann M, Birch S, Kuenneth C, Nuovo J, Azari R, Oto-Kent D, Rooney M. Recruitment strategies for minority participation: challenges and cost lessons from the POWER interview. Ethn Dis. 2005;15(3):395–406. [PubMed] [Google Scholar]
- 46.Swanson GM, Ward AJ. Recruiting minorities into clinical trials toward a participant-friendly system. J Natl Cancer Inst. 1995;87(23):1747. doi: 10.1093/jnci/87.23.1747. [DOI] [PubMed] [Google Scholar]