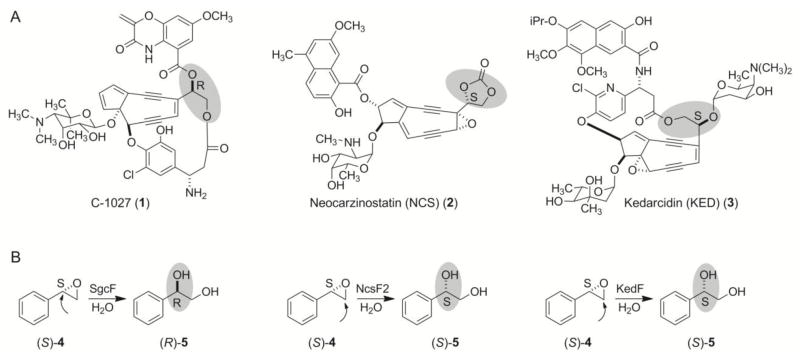
Figure 1.
(A) Structures of the enediyne chromophores of chromoproteins C-1027 (1), neocarzinostatin (NCS, 2), and kedarcidin (KED, 3), with respective (R)- and (S)-vicinal diol stereochemistry highlighted. (B) The stereochemical outcome from hydrolysis of (S)-4 catalyzed by SgcF, NcsF2, and KedF, the respective EHs from the 1, 2, and 3 biosynthetic machinery. Arrows indicate the regioselectivity of epoxide ring opening in each enzyme reaction.