Figure 3.
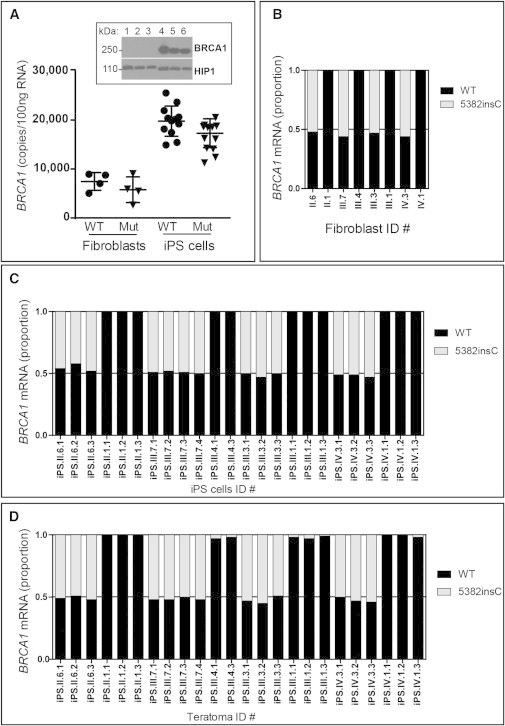
BRCA1 Wild-Type and Mutant Expression in Fibroblasts, iPSCs, and Teratomas
(A) qPCR for absolute BRCA1 mRNA levels in wild-type (WT) and BRCA1 5382insC fibroblasts and iPSCs. Reprogramming led to a doubling of total BRCA1 mRNA in iPSCs. A total of eight fibroblast cell lines and 24 iPSC lines were analyzed. Each point is an average of three replicate PCR reactions. The difference in expression of BRCA1 between the iPSCs and fibroblasts was statistically significant (Pearson chi squared, p < 0.001). The western blot for BRCA1 (top panel) in six BRCA1 WT fibroblast (lanes 1–3) or iPSC (lanes 4–6) lines confirmed that the BRCA1 protein is also induced with reprogramming. The HIP1 protein was used as a loading control and migrates faster when derived from iPSC extracts compared with fibroblast extracts (bottom panel).
(B) Allele-specific qPCR was used to quantitate the relative levels of WT (black bar) and mutant (gray bar) BRCA1 mRNA in fibroblasts. The eight fibroblast cell lines were analyzed with three replicate reactions and averaged. There were no significant differences in expression of the WT and mutant alleles.
(C) qPCR, as in (B), was used to quantitate the relative levels of WT (black bar) and mutant (gray bar) BRCA1 mRNA in iPSC lines. The 24 iPSC lines were analyzed with three replicate reactions and averaged. There were no significant differences in expression of the WT and mutant alleles.
(D) qPCR, as in (B), was used to quantitate the relative levels of WT (black bar) and mutant (gray bar) BRCA1 mRNA in teratomas. RNAs from the 24 teratomas were analyzed with three replicate reactions and averaged. There were no significant differences in expression of the WT and mutant alleles.
See also Figure S4.
