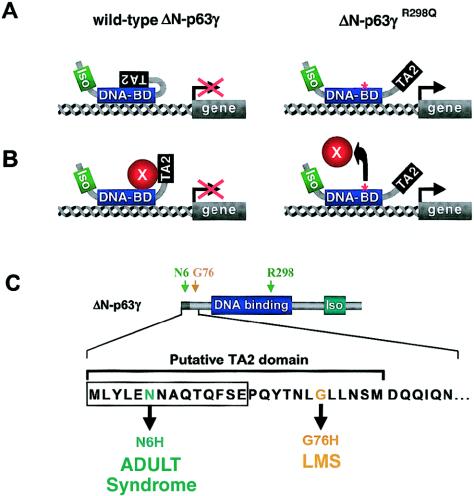
Figure 4.
A gain-of-function mutation in ADULT syndrome reveals a second TA domain (TA2) in p63. A and B, Models explaining the gain-of-function effect that the R298Q mutation has on the ΔN-p63γ isotype, which normally does not posses transactivation. The TA2 domain is normally kept in an inactive state, either because of intramolecular interaction (A) or because of binding of another protein (B). This inhibition is proposed to be released in patients with ADULT syndrome because the R298Q mutation abolishes this protein-protein interaction. DNA-BD = DBD; Iso = ISO domain; TA2 = TA2 domain. C, Position of TA2 domain, as determined by Dohn et al. (2001). The TA2 domain consists of 14 amino acids specific to the ΔN isotypes and 12 amino acids common to all p63 isotypes. Interestingly, another ADULT-syndrome mutation and an LMS mutation both give rise to amino acid substitutions within the TA2 domain. DNA binding = DBD; Iso = ISO domain.