Figure 1.
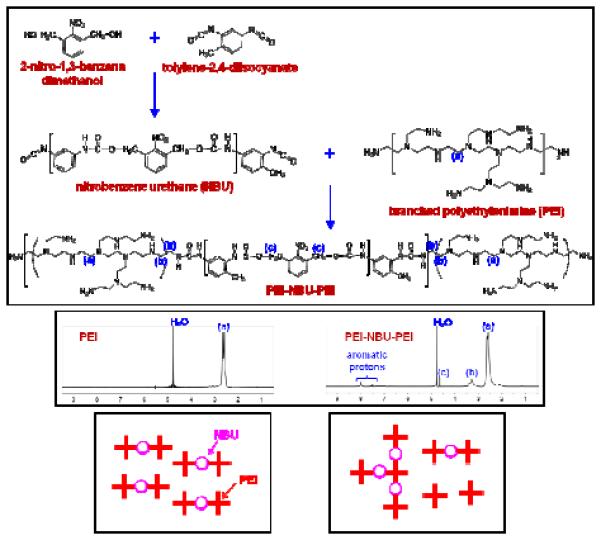
(Upper) Synthesis route for the preparation of a UV-degradable crosslinked polyethylenimine (PEI) material (named “ENE4-1”). Low molecular weight branched PEI (bPEI-2k) polymers were crosslinked with a UV-cleavable o-nitrobenzyl urethane (NBU) spacer. (Middle) 1H NMR spectra of the bPEI-2k precursor and the PEI-NBU-PEI product in D2O. In the synthesis of the ENE4-1 material, bPEI-2k was reacted with NBU in a 2:1 stoichiometric molar ratio. As the bottom right cartoon describes, the random nature of the coupling reaction results in a polydisperse product (rather than a monodisperse product such as shown in bottom left of the figure).
