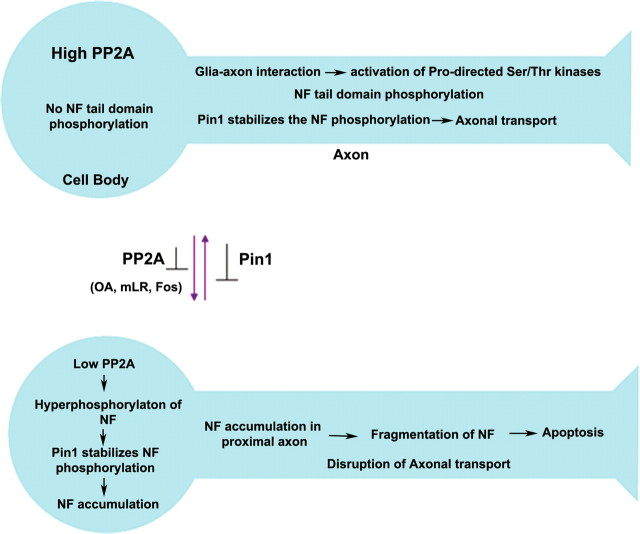
Figure 12.
Role of Pin1 in the progression of compartment-specific NF-M/H phosphorylation. Top, In normal neurons, the NF phosphorylation is selective in the axonal compartment in normal neurons. After NF protein synthesis, higher activity of PP2A in cell bodies inhibits the NF tail domain phosphorylation. Although Pin1 is localized in cell bodies, it cannot act on the non-S/T-P phosphorylated proteins. During assembly of NF in axonal hillock region, NFs are transported by slow axonal transport. In the axonal compartment, the axonal–glial interaction activates the Ser/Thr kinases and phosphorylates some of pSer/Thr-Pro residues. The phosphorylation of these residues renders the phospho-Ser/Thr-Pro to remain in cis configuration and blocks additional phosphorylation. However, Pin1 induces cis-trans isomerization, increases the accessibility of the Ser/Thr kinases, and stabilizes the NF phosphorylation. Because of the high PP2A activity, there is no NF phosphorylation in the cell bodies. Bottom, Inhibition of PP2A activity leads to aberrant perikaryal hyperphosphorylation of NF proteins in cell bodies. Pin1 stabilizes the NF phosphorylation in the cell bodies, phospho-NF proteins are accumulated in the cell bodies and proximal axons, and NF transport is impaired. This is consistent with the observation that PP2A activity is reduced in AD brain and ALS spinal cord with higher accumulations of phospho-NF in the perikarya.