ABSTRACT
BACKGROUND:
Pancreatic cancer is the fourth leading cause of cancer deaths in the United States. A minority of patients present with localized disease and surgical resection still offers patients the only hope for long-term survival. Locally advanced pancreatic cancer is defined as surgically unresectable, but has no evidence of distant metastases. The purpose of this study is to evaluate the efficacy and safety of cetuximab in combination with gemcitabine and 5-FU along with radiation therapy in locally advanced non-resectable, pancreatic adenocarcinoma, using progression free survival as the primary end point.
METHODS:
This was a prospective, single arm, open label pilot phase II study to evaluate the anti-tumor activity of gemcitabine (200 mg/m2 per week) and cetuximab (250 mg/m2 per week after an initial 400 mg/m2 loading dose) with continuous infusion 5-FU (800 mg/m2 over 96 hours) and daily concurrent external beam radiation therapy (50.4 Gy total dose) for six weeks (cycle 1) in patients with non-metastatic, locally advanced pancreatic adenocarcinoma. Following neoadjuvant treatment, subjects were re-evaluated for response and surgical candidacy with restaging scans. After resection, or also if not resected; subjects received further therapy with four 28-day cycles (cycles 2-5) of weekly gemcitabine (1000 mg/m2) and cetuximab (250 mg/m2) on days 1, 8, and 15.
RESULTS:
Between 2006 and 2011, twenty-six patients were screened and eleven of them were enrolled in the study. Most common reasons for screen failures were having resectable disease, metastatic disease or co-morbidity. Ten patients were able to tolerate and complete cycle 1 of chemoradiotherapy. One patient stopped the study prematurely due to grade III diarrhea. All except this one patient received planned radiation therapy. The response evaluation after cycle 1 showed one Partial Response, eight Stable Disease and two Progressive Disease. Four patients subsequently underwent surgical resection of the tumor. All patients had R0 resections. There was one preoperative mortality due to multiple organ failure. Median progression free survival (PFS) for four resected patients was 9.0 months while for unresected patients median PFS was 7.1 months. Median overall survival (OS) for four resected patients was 47.4 months and for unresected patients median OS was 17.0 months. Most common adverse events were hematologic (27%). Only two patients developed grade 3 neutropenia. Most common treatment related non-hematologic adverse events were diarrhea (10 of 11), nausea (8 of 11) and skin rash (10 of 11 patients). Only 9.5% of all reported non-hematologic adverse events were grade 3 or higher.
CONCLUSIONS:
The combination of cetuximab, weekly gemcitabine and continuous infusion of 5-FU with radiotherapy was quite well tolerated with intriguing clinical benefit and survival results in carefully selected patients with locally advanced pancreatic adenocarcinoma. A trial with larger sample size will be necessary to confirm these results.
Pancreatic cancer is the fourth leading cause of cancer deaths in the United States.1 The overall 5-year survival rate among patients with pancreatic cancer is <5%.2,3 A minority of patients present with localized disease, and surgical resection still offers them the only hope for long-term survival. Unfortunately, only 5% to 25% of patients present with tumors amenable to resection.4
Locally advanced pancreatic cancer is defined as surgically unresectable, but has no evidence of distant metastases. In this study, a tumor was considered to be unresectable if it had one of the following features: extensive peripancreatic lymph node involvement; encasement or occlusion of the superior mesenteric vein (SMV) or SMV/portal vein confluence; or direct involvement of the superior mesenteric artery (SMA), celiac axis, inferior vena cava, or aorta.
Currently, there is no consensus on the treatment of locally advanced disease. Most common treatment options include external beam radiation therapy (EBRT), alone or combined with chemotherapy. 5-Fluorouracil (5-FU)- and gemcitabine-based regimens have been studied extensively, since both are radiosensitizing agents. With treatment, median survival of patients with locally advanced disease is limited to 10 to 12 months.5 In evaluating the results of various therapies, it is useful to remember that, in this subset of the population, progression-free survival (PFS) has been very modest (4–5 months).6
Targeted therapy with signal transduction inhibitors appears to represent a major step forward in the treatments of cancer.7 EGFR receptors seem to play a particularly important role in human carcinogenesis, and EGFR inhibitors (EGFRi) are in clinical use in lung cancer and colon cancer. A phase III trial demonstrated promising activity against advanced pancreatic cancer with the EGFRi erlotinib, when combined with gemcitabine.8
Because of poor prognosis and lack of survival benefit, surgery has generally not been considered as a part of management in locally advanced disease. However, a recent meta-analysis of prospective studies indicated a potential advantage for a minority of those with unresectable lesions.9
The purpose of this study was therefore to evaluate the efficacy and safety of cetuximab in combination with gemcitabine and 5FU, along with radiation therapy, in locally advanced, nonresectable, pancreatic adenocarcinoma, using PFS as the primary end point. Patients were also evaluated for resectability after treatment.
PATIENTS AND METHODS
Patient Population
This was a prospective, single-arm, pilot phase II study to evaluate the antitumor activity of gemcitabine (200 mg/m2 per week) and cetuximab (250 mg/m2 per week after an initial 400-mg/m2 loading dose) when given with continuous infusion 5-FU (800 mg/m2 over 96 hours by continuous infusion Monday through Friday) and daily concurrent EBRT (50.4-Gy total dose) in patients with nonmetastatic, locally advanced, unresectable, or borderline-resectable pancreatic carcinoma. The patients were evaluated for resection after chemoradiotherapy.
Patients with unresectable or borderline-resectable pancreatic adenocarcinoma were eligible for the study. The University of Massachusetts Medical School Institutional Review Board (Worcester, MA) approved the study protocol, and all patients gave written, informed consent. Before study treatment, to determine eligibility, all patients underwent computed tomography (CT) of the abdomen and pelvis, CT or x-ray of the chest, endoscopic ultrasound (EUS), and, for tumor staging, either laparoscopy or laparotomy. Only patients with histologic confirmation of a diagnosis of pancreatic adenocarcinoma who had measurable disease per RECIST (Response Evaluation Criteria in Solid Tumors) criteria, with locoregional disease not amenable to surgery (unresectable or borderline resectable) on the basis of 1 or more of the following CT/EUS criteria, were enrolled: (1) size of the tumor, >5 cm; (2) lymph nodes (bulky, >2 cm, but within radiation port); (3) vascular involvement or impingement on major vessels (SMA, SMV, portal vein, or hepatic artery); and (4) invasion into the adjacent structures.
Other eligibility criteria included ECOG (Eastern Cooperative Oncology Group) status 0 or 1 at baseline, adequate renal function (serum creatinine, ≤2 mg/dL); bilirubin, ≤2 mg/dL; AST and ALT, <3 times the upper limits of normal; and adequate blood counts (WBC, >3,000/mm3; ANC, >1,500/mm3; and platelets, >100,000 mm3).
Patients were excluded if they had resectable disease at baseline; had prior therapy for pancreatic adenocarcinoma; had uncontrolled, concurrent, serious medical or psychiatric illness; or had experienced myocardial infarction in the past 6 months.
Chemoradiation Cycle 1: Neoadjuvant
In the first cycle, for 6 weeks, all the participants were given gemcitabine (200 mg/m2 per week) and cetuximab (250 mg/m2 per week, after an initial 400-mg/m2 loading dose) along with 5-FU (800 mg/m2 over 96 hours by continuous infusion, Monday through Friday) and daily concurrent EBRT (50.4-Gy total dose). Patients were evaluated for resection after chemoradiotherapy. The study schema is shown in Figure 1.
Figure 1.
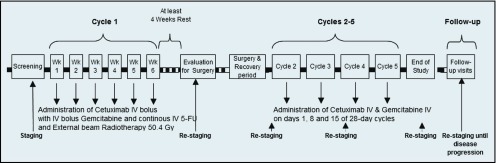
The treatment plan.
Evaluation of Response and Surgery
After cycle 1, patients were allowed a rest period (minimum, 4 weeks; maximum, 3 months). The tumors were restaged with abdominopelvic CT and chest CT or x-ray at 4 weeks after treatment and re-evaluated for resectability. If surgery was performed, a minimum 4-week recovery period was necessary before cycle 2 began. If the tumor was resected, standardized histologic evaluation of the specimen was performed by a gastrointestinal pathologist. The surgical margins of the tumors were recorded as negative (R0) or positive (R1).
Even if surgery was not performed, a minimum 4-week rest period was allowed before cycle 2 began, with a maximum rest period of 3 months after completion of cycle 1.
Chemoradiation Cycles 2 to 5
After tumor resection, or if no resection was performed, patients received further therapy with four 28-day cycles of weekly gemcitabine (1,000 mg/m2 on days 1, 8, and 15) and cetuximab (250 mg/m2 on days 1, 8, and 15). Restaging with abdominopelvic CT and chest CT or x-ray was performed before day 1 of cycles 2 and 4.
Requirements During Treatment and Follow-up
A physical examination including vital signs, ECOG (Eastern Cooperative Oncology Group) performance status, and weight was performed weekly. Weekly assessments also included evaluation and documentation of adverse events and concomitant medications. Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria, version 3 (CTCAE, v3.0). Blood counts, along with serum chemistries including renal, liver function, calcium, and magnesium, were performed weekly at each treatment visit while the patient was on active therapy and for the immediate periods after cycles 1 and 5, as clinically indicated. Serum CA19-9 was checked on day 1 of each cycle.
Chemotherapy doses were held or reduced using standard dose-modification tables for hematologic and nonhematologic toxicities. For skin toxicities related to cetuximab, the manufacturer's dose modifications were followed. All subjects were required to receive doxycycline (100 mg orally twice a day) to prevent a severe, cetuximab-related rash. During cycle 1, chemotherapy and radiation therapy were postponed if the patient's ANC (absolute neutrophil count) was less than 1,000 cells/mm3 or if the platelets were less than 75,000 cells/mm3. During cycle 2 and onward, chemotherapy was postponed if the patient's ANC was less than 1,000 cells/mm3 or if the platelets were less than 50,000 cells/mm3. Doses of chemotherapy that were reduced were not re-escalated for the remainder of the cycles. If treatment was postponed for greater than 3 weeks, the subject was removed from the protocol treatment.
An end-of-therapy visit was conducted within 4 weeks after administration of the last dose of chemotherapy. The patients underwent a physical examination, including vital signs and CA19-9 level. Repeat response evaluations were also performed with abdominopelvic CT and chest CT (or chest x-ray).
The subjects were evaluated every 3 months for 1 year, then every 6 months for an additional 2 years or until progression, by physical examination, abdominopelvic CT and chest CT (or chest x-ray), and laboratory studies.
RESULTS
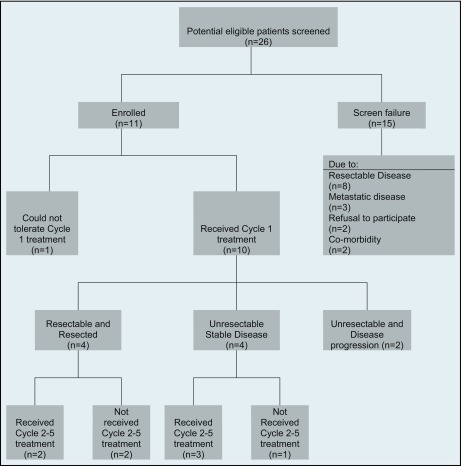
Between June 2006 and March 2011, 26 patients were screened, and 11 of them were enrolled in this study. The flow of 26 patients through the treatment protocol, regardless of their disease status, is shown in Figure 2.
Figure 2.
Algorithm of the flow of patients through the treatment protocol.
The most common reasons for screening failure were resectable disease and detection of metastatic lesions. Characteristics for study subjects and those who failed screening are listed in Table 1. Of the 11 subjects enrolled, 10 were able to complete neoadjuvant treatment cycle 1 successfully with minimal and tolerable side effects.
Table 1.
Patient characteristics for study subjects and screening failures
| Study subjects (n = 11) | Screening failures (n = 15) | |
|---|---|---|
| Age | ||
| Mean (years) | 63.5 | 64.5 |
| Median (years) | 66 (range: 48-78) | 65 (range: 43-78) |
| Sex, n (%) | ||
| Male | 7 (64) | 4 (27) |
| Female | 4 (36) | 11 (73) |
| Performance status, n (%) | ||
| 0 | 5 (45) | 7 (47) |
| 1 | 6 (55) | 8 (53) |
| Race n (%) | ||
| White | 10 (91) | 12 (80) |
| Black | 0 | 0 |
| Hispanic | 1 (9) | 1 (7) |
| Asian | 0 | 2 (13) |
| Tumor characteristics, n (%) | ||
| Localized resectable | 0 | 8 (53) |
| Borderline resectable | 4 (36) | 0 |
| Locally advanced unresectable | 7 (64) | 4 (27) |
| Metastatic | 0 | 3 (20) |
| Stage n (%) | ||
| 1 | 0 | 4 (27) |
| 2 | 5 (45) | 5 (33) |
| 3 | 6 (55) | 3 (20) |
| 4 | 0 | 3 (20) |
| Primary pancreatic site, n (%) | ||
| Head | 6 (55) | 12 (79) |
| Head and body | 1 (9) | 1 (7) |
| Head, body and tail | 1 (9) | 0 |
| Body | 3 (27) | 1 (7) |
| Body and tail | 0 | 1 (7) |
| Tail | 0 | 0 |
Ten patients were able to tolerate and complete cycle 1 of chemoradiotherapy. Doses of chemotherapy needed modification in 10 patients, most commonly because of hematologic toxicities. One patient withdrew from the study prematurely because of grade 3 diarrhea. All except that patient received the planned radiation therapy. The response evaluation per RECIST criteria after the cycle 1 showed: 1 partial response, 8 stable disease, and 2 disease progression. Subjects' baseline characteristics, treatments received, and their overall responses to treatment are presented in Table 2.
Table 2.
Subject's baseline characteristics and their response to treatment
| Treatment Received |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Age at Diagnosis | Sex | Stage | ECOG* | Surgical Evaluation | Surgery Received | Cycle 1 | Cycle 2–5 | CA19-9 Response† | Radiologic Response‡ | PFS§ | Overall Survival‖ | Survival Status‖ |
| 1 | 76 | Male | IIa | 0 | Borderline resectable | Yes | Yes | Yes | Yes | SD | 15.9 | 65.6 | Alive |
| 2 | 56 | Male | III | 0 | Locally advanced | No | Yes | No | Yes | SD | Data not available | 15.4 | Deceased |
| 3 | 72 | Male | IIa | 1 | Borderline resectable | Yes | Yes | No | Yes | SD | 9.0 | 55.5 | Alive |
| 4 | 67 | Male | IIa | 0 | Locally Advanced | No | Yes | Yes | Yes | SD | 18.4 | 27.7 | Deceased |
| 5 | 59 | Female | IIa | 0 | Borderline resectable | Yes | Yes | Yes | No | SD | Data not available | 39.2 | Alive |
| 6 | 57 | Male | III | 1 | Locally advanced | No | Yes | Yes | Yes | SD | 7.3 | 15.7 | Deceased |
| 7 | 52 | Female | III | 1 | Locally advanced | Yes | Yes | No | Yes | PR | 4.9 | 4.9 | Deceased |
| 8 | 66 | Female | IIa | 1 | Borderline resectable | No | Yes | No | Yes | PD | 4.2 | 19.5 | Deceased |
| 9 | 48 | Male | III | 0 | Locally advanced | No | Yes | No | No | PD | 2.5 | 17.1 | Deceased |
| 10 | 78 | Female | III | 1 | Locally advanced | No | No | No | Yes | SD | 7.2 | 7.2 | Deceased |
| 11 | 68 | Male | III | 1 | Locally Advanced | No | Yes | Yes | Yes | SD | No progression | 20.6 | Alive |
ECOG performance status.
CA19-9 Response defined as more than 20% decrease in serum level.
Per Response Evaluation Criteria in solid Tumors (RECIST) Committee (J Natl Cancer Inst 92:205–216, 2000).
Progression-free survival in months.
Overall survival in months. Survival status as of July 13, 2012.
Four subjects underwent resection after cycle 1 neoadjuvant treatment. All had clear margins with R0 resection. Among them, subject 7, who had unresectable, locally advanced disease, had partial response per RECIST after neoadjuvant treatment. This subject had perioperative complications, such as massive blood loss and multiorgan failure, and died within 6 weeks after a prolonged ICU stay after surgery. The remaining 3 patients had no early or late surgical complications.
The average time from completion of cycle 1 neoadjuvant treatment to surgery in 4 patients who had resection was 8.3 (range, 6.7–9.8) weeks. Average time from surgery to initiation of cycle 2 treatment in 2 patients who started and completed cycles 2 to 5 was 8.6 (range, 7.5–9.7) weeks.
Eight subjects (73%) had CA19-9 response, defined as at least a 20% decrease from baseline, either at the end of the first cycle or at the end of planned treatment. Six subjects started cycle 2, but 5 completed all planned cycles 2 to 5.
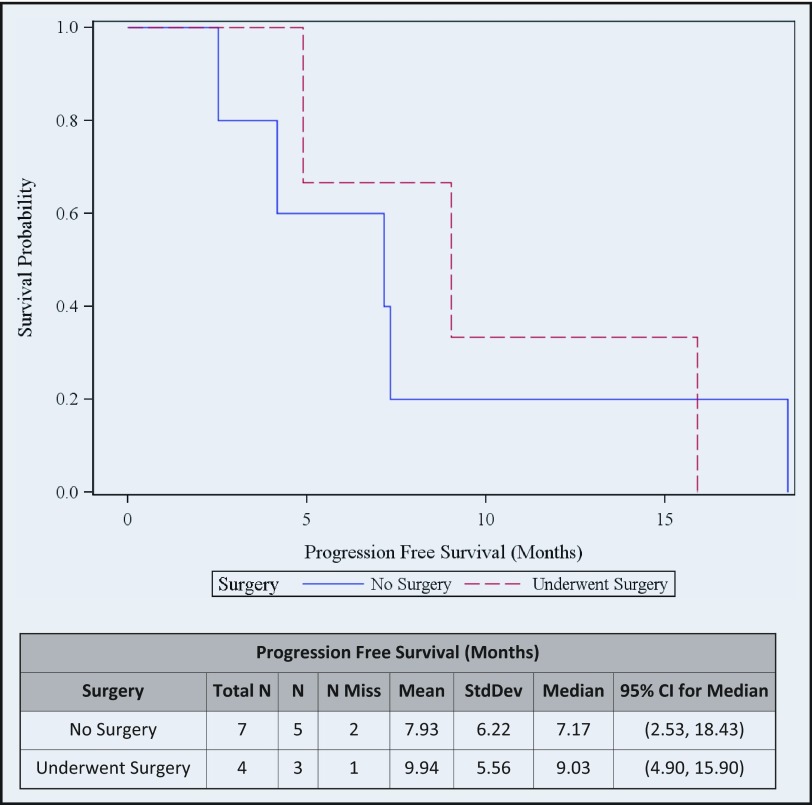
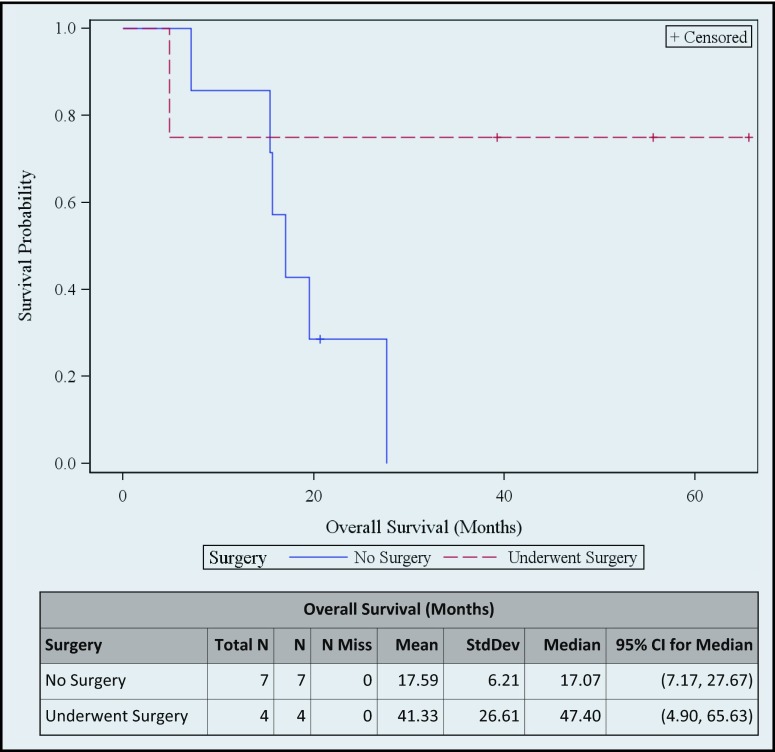
The median progression-free survival (PFS) of 4 patients with resected tumor was 9.0 months (95% CI, 9.0–15.9), whereas for those with unresected tumor, the median PFS was 7.1 months (95% CI, 2.5–18.4). Median overall survival (OS) of the 4 patients with resected tumor was 47.4 months (95% CI, 4.9–65.6) and for those with unresected tumor, median OS was 17.0 months (95% CI, 7.1–27.6) (Figure 3 and Figure 4).
Figure 3.
Progression-free survival.
Figure 4.
Overall survival.
Overall, most common adverse events were hematologic (27%). Only 2 patients developed grade 3 neutropenia at some point. Most common treatment-related nonhematologic adverse events were diarrhea (10/11 patients), nausea (8/11), and skin rash (10/11). Only 9.5% of all reported nonhematologic adverse events were grade 3 or higher.
Toxicities reported during cycle 1 chemoradiation therapy are listed in Table 3. During this phase of the study 207 adverse events were observed, with 18.7% due to hematoxicity. Hematologic toxicities were mostly grade 1 and 2. Severe (grade 3 or 4) neutropenia or thrombocytopenia were not observed during cycle 1. Most common treatment-related nonhematologic adverse events were diarrhea, nausea, and skin rash. Grades 3 and 4 nonhematologic toxicities constituted 10.1% of all nonhematologic toxicities and were observed in 6 patients. Hospitalization was required in 4 patients during cycle 1 because of severe diarrhea that resulted in electrolyte disturbance and cardiac rhythm disorders (1 patient), nausea (1 patient), radiation-related duodenitis and biliary obstruction (1 patient), and dehydration and biliary obstruction (1 patient).
Table 3.
Toxicity Assessment during cycle 1 neoadjuvant chemoradiation therapy
| Number of patients (n = 11) |
Number of events (n = 207) |
|||||
|---|---|---|---|---|---|---|
| Adverse Event | Total | Grade 1–2 | Grade 3–4 | Total | Grade 1–2 | Grade 3–4 |
| Hematologic | 11 | 11 | 6 | 39 | 32 | 7 |
| Anemia | 1 | 1 | 0 | 1 | 1 | 0 |
| Lymphopenia | 10 | 7 | 6 | 17 | 10 | 7 |
| Neutropenia | 5 | 5 | 0 | 8 | 8 | 0 |
| Thrombocytopenia | 7 | 7 | 0 | 13 | 13 | 0 |
| Nonhematologic | 11 | 11 | 6 | 168 | 151 | 17 |
| Abdominal pain | 3 | 3 | 0 | 4 | 4 | 0 |
| ALP elevated | 5 | 5 | 1 | 7 | 6 | 1 |
| ALT elevated | 4 | 3 | 1 | 6 | 5 | 1 |
| Anorexia | 5 | 3 | 2 | 5 | 3 | 2 |
| Arthralgia | 2 | 2 | 0 | 2 | 2 | 0 |
| AST elevated | 4 | 4 | 0 | 5 | 5 | 0 |
| Biliary obstruction | 2 | 0 | 2 | 2 | 0 | 2 |
| Bilirubin elevated | 3 | 1 | 2 | 3 | 1 | 2 |
| Constipation | 4 | 4 | 0 | 4 | 4 | 0 |
| Cough | 1 | 1 | 0 | 2 | 2 | 0 |
| Diarrhea | 8 | 7 | 1 | 17 | 16 | 1 |
| Dizziness | 2 | 2 | 0 | 2 | 2 | 0 |
| Dry skin | 5 | 5 | 0 | 5 | 5 | 0 |
| Edema, lower extremities | 2 | 2 | 0 | 2 | 2 | 0 |
| Fatigue | 5 | 5 | 0 | 6 | 6 | 0 |
| Fever | 2 | 2 | 0 | 6 | 6 | 0 |
| Heartburn | 2 | 2 | 0 | 2 | 2 | 0 |
| Hypoalbuminemia | 8 | 8 | 0 | 10 | 10 | 0 |
| Hypocalcemia | 8 | 8 | 0 | 10 | 10 | 0 |
| Hypokalemia | 5 | 4 | 2 | 8 | 6 | 2 |
| Hyponatremia | 2 | 2 | 1 | 3 | 2 | 1 |
| Hypotension | 2 | 2 | 0 | 2 | 2 | 0 |
| Mucositis | 2 | 2 | 0 | 2 | 2 | 0 |
| Nasal bleed | 2 | 2 | 0 | 2 | 2 | 0 |
| Nausea | 7 | 7 | 0 | 10 | 10 | 0 |
| Pruritus | 2 | 2 | 0 | 2 | 2 | 0 |
| Skin rash | 9 | 9 | 0 | 9 | 9 | 0 |
| Vomiting | 5 | 4 | 1 | 6 | 5 | 1 |
| Weight Loss | 4 | 4 | 0 | 4 | 4 | 0 |
| Other | 9 | 7 | 3 | 20 | 16 | 4 |
The toxicity assessment for cycles 2 to 5 includes all 6 patients who started cycle 2. The toxicities observed during cycles 2 to 5 are outlined in Table 4. Seventy-four adverse events were observed during this phase of the study. Hematologic toxicities were common and were seen in all patients; however, grade 3 to 4 hematologic toxicities were rare. The most common treatment-related nonhematologic toxicities were diarrhea, skin rash, and elevated liver enzymes. Hospitalization was required for only 1 patient who had severe abdominal pain.
Table 4.
Toxicity assessment during cycles 2 to 5 of chemotherapy
| Number of patients (n = 6) |
Number of events (n=74) |
|||||
|---|---|---|---|---|---|---|
| Adverse Event | Total | Grade 1–2 | Grade 3–4 | Total | Grade 1–2 | Grade 3–4 |
| Hematologic | 6 | 6 | 4 | 38 | 27 | 11 |
| Anemia | 4 | 3 | 2 | 5 | 3 | 2 |
| Lymphopenia | 5 | 2 | 4 | 10 | 4 | 6 |
| Neutropenia | 6 | 6 | 2 | 13 | 10 | 3 |
| Thrombocytopenia | 5 | 5 | 0 | 10 | 10 | 0 |
| Nonhematologic | 6 | 6 | 2 | 41 | 38 | 3 |
| Abdominal pain | 3 | 2 | 1 | 3 | 2 | 1 |
| ALP elevated | 1 | 1 | 0 | 1 | 1 | 0 |
| ALT elevated | 3 | 3 | 0 | 3 | 3 | 0 |
| Anorexia | 1 | 1 | 0 | 1 | 1 | 0 |
| Ascites | 1 | 1 | 0 | 1 | 1 | 0 |
| AST elevated | 3 | 3 | 0 | 3 | 3 | 0 |
| Diarrhea | 3 | 3 | 0 | 6 | 6 | 0 |
| Dizziness | 2 | 2 | 0 | 2 | 2 | 0 |
| Fatigue | 1 | 1 | 0 | 1 | 1 | 0 |
| Hematochezia | 1 | 1 | 0 | 1 | 1 | 0 |
| Hypoalbuminemia | 1 | 1 | 0 | 1 | 1 | 0 |
| Hypocalcemia | 1 | 1 | 0 | 1 | 1 | 0 |
| Hypokalemia | 3 | 3 | 0 | 3 | 3 | 0 |
| Mood alteration | 1 | 1 | 0 | 1 | 1 | 0 |
| Nasal bleed | 2 | 2 | 0 | 2 | 2 | 0 |
| Nausea | 1 | 1 | 0 | 1 | 1 | 0 |
| Pruritus | 1 | 1 | 0 | 1 | 1 | 0 |
| Skin fissures | 1 | 1 | 0 | 1 | 1 | 0 |
| Skin rash | 3 | 3 | 1 | 4 | 3 | 1 |
| Vomiting | 1 | 1 | 0 | 1 | 1 | 0 |
| Weight loss | 2 | 1 | 1 | 2 | 1 | 1 |
| Wheezing | 1 | 1 | 0 | 1 | 1 | 0 |
DISCUSSION
The most recent review of chemoradiation in locally advanced disease showed that, according to the data from randomized clinical trials, chemoradiation regimens achieved a median overall survival of 6.1 to 14.5 months and PFS of 2.7 to 13 months.10
There is insufficient evidence to endorse the use of chemoradiation followed by chemotherapy over chemotherapy alone in locally advanced disease.11 However a retrospective study using the Surveillance, Epidemiology, and End Results (SEER) Medicare database showed chemoradiation to be superior to radiation therapy alone, chemotherapy alone, or no treatment, with adjusted mean survivals of 47, 29, 27, and 15 weeks, respectively.12
In our study, the combination of cetuximab, weekly gemcitabine, and infusional 5-FU with radiotherapy was quite well tolerated with interesting clinical benefit and survival results. The study was terminated prematurely because of low enrollment, and it was not possible to establish statistically significant results comparable to those in the literature because of the combination of patients with borderline-resectable tumor and those with unresectable tumor.
Overall survival is found to be more than 47 months for those with resected tumor and 17 months for those with unresected tumor, perhaps because all 4 patients who underwent surgery had R0 resections. One patient (subject 11) who could not undergo surgery after cycle 1 neoadjuvant treatment, finished all the planned cycles and continued the treatment with cetuximab and gemcitabine off protocol, and he has been free of disease progression for more than 20 months. On the other hand, two patients (subjects 8 and 9) developed disease progression with metastatic lesions immediately after cycle 1 neoadjuvant treatment. Most of the patients who have the highest survival rates had excellent performance status at diagnosis and achieved CA19-9 response at some point during their treatment.
In their large prospective cohort, Katz et al13 treated 160 borderline-resectable tumors with neoadjuvant chemotherapy, chemoradiation, or both. They found that 66 patients who completed all therapy including resection had a median survival of 40 months whereas 94 patients who did not undergo resection had a median survival of only 13 months.
Mamon et al .14 conducted a very similar study, a phase 2 trial in which cycle 1 consisted of radiation at 50.4 Gy in 28 fractions over 5.5 weeks, with 5FU given as a continuous infusion from Monday through Friday at 200 mg/m2/day and gemcitabine given weekly at 200 mg/m2; both were given throughout the radiation therapy course. Three weeks after the completion of radiation, patients received gemcitabine at a dose of 1,000 mg/m2 over 30 minutes weekly for 3 weeks, followed by a 1-week rest, for four 4-week cycles. Surgery was not an option for this patient group. Median overall survival of 78 subjects was reported as 12.2 months. The regimen was well tolerated.
With the addition of cetuximab, our treatment regimen suggests a better outcome and could offer an alternative treatment for select patients. However a trial with a larger sample size is necessary to confirm the results and compare them with those in the literature. Correlative studies should also be included to explain the variable patient responses to this treatment regimen.
REFERENCES
- 1. American Cancer Society Cancer Facts & Figures 2010. Atlanta: American Cancer Society, 2010 [Google Scholar]
- 2. Li D, Xie K, Wolff R, et al. : Pancreatic cancer. Lancet 363:1049–1057, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Hidalgo M: Pancreatic Cancer. N Engl J Med 362:1605–1617, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Willett CG, Czito BG, Bendell JC, et al. : Locally advanced pancreatic cancer. J Clin Oncol 23:4538–44, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Chang BW, Saif MW: Locally Advanced Pancreatic Adenocarcinoma: Where Are We and Where Are We Going? J Pancreas (Online) 12:101–105, 2011 [PubMed] [Google Scholar]
- 6. Blackstock AW, Tepper JE, Niedwiecki D, et al. : Cancer and Leukemia Group B (CALGB) 89805: Phase II chemoradiation trial using gemcitabine in patients with locoregional adenocarcinoma of the pancreas. Int J Gastrointest Cancer 34:107–116, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Xiong Henry Q., Arthur Rosenberg, et al. : Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II trial. J Clin Oncol Vol 22:2610–2616, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Moore MJ, Goldstein D, Hamm J, et al. : Erlotinib improves survival when added to gemcitabine in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group [NCIC-CTG]. ASCO Gastrointestinal Cancers Symposium, Miami, FL, January 27-29, 2005 (abstr 77) [Google Scholar]
- 9. Andriulli A, Festa V, Botteri E, et al. : Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analysis of prospective studies. Ann Surg Oncol 19:1644–1662, 2012 [DOI] [PubMed] [Google Scholar]
- 10. Huguet F, Girard N, Guerche CS, et al. : Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol 27:2269–2277, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Sultana A, Tudur Smith C, Cunningham D, et al. : Systematic review, including meta-analyses, on the management of locally advanced pancreatic cancer using radiation/combined modality therapy. Br J Cancer 96:2269–1183, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krzyzanowska MK, Weeks JC, Earle CC: Treatment of locally advanced pancreatic cancer in the real world: population-based practices and effectiveness. J Clin Oncol 21:3409–3414, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Katz MH, Pisters PW, Evans DB, et al. : Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 206:833–846, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mamon HJ, Niedzwiecki D, Hollis D, et al. : A phase 2 trial of gemcitabine, 5-fluorouracil, and radiation therapy in locally advanced nonmetastatic pancreatic adenocarcinoma: Cancer and Leukemia Group B (CALGB) 80003. Cancer 117:2620–2628, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]