Figure 5.
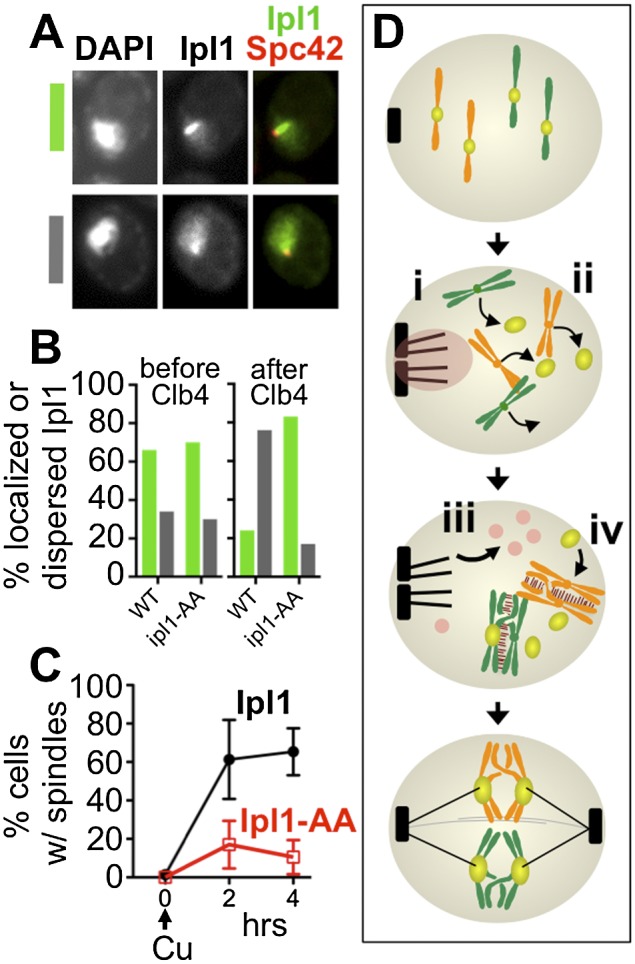
Phosphorylation of Ipl1 is necessary to trigger precocious spindle assembly in prophase. To test whether CDK phosphorylation of Ipl1 controls its localization in meiotic prophase, prophase-arrested cells (ndt80Δ) expressing either Ipl1-GFP (DHC257) or nonphosphorylatable Ipl1-AA-GFP (DHC258 and DHC259) were treated with copper to induce expression of CLB4 (PCUP1-CLB4) and then monitored to follow Ipl1 localization and spindle formation (Spc42-DSRed). (A) Three hours after meiotic entry (before CLB4 induction), some cells exhibited intense localization to the microtubule array (green), while others had a more dispersed nuclear distribution (gray). (B) Cells were assayed for their Ipl1 distribution at the time of copper addition (3 h after introduction to sporulation medium) or 4 h later. Following CLB4 induction, Ipl1-AA-GFP showed significantly higher levels of cells with Ipl1 at the microtubule array (n = 100 for both samples; Fisher's exact test, P > 0.0001). (C) Spindle formation (Spc42-DSRed) was monitored following CLB4 induction in cells expressing Ipl1-GFP (black) or Ipl1-AA-GFP (red) (average ± one standard deviation of three experiments). (D) Model for the coordination of microtubule and chromosome behaviors by Ipl1 and Ndt80 in meiosis. Upon meiotic entry, Ipl1 localizes to SPBs (i), preventing their separation, and triggers shedding of Ndc80 (ii). When homologous chromosomes have successfully partnered, Ndt80 activation triggers increased CDK activity (iii), which results in delocalization of Ipl1 from SPBs, and promotes kinetochore reassembly through an unknown mechanism (iv).
