Abstract
Repopulation of memory T cells (Tmem) in allograft recipients after lymphodepletion is a major barrier to transplant tolerance induction. Ineffective depletion of naïve T cells (Tn) and Tmem may predispose to repopulation of Tmem after transplantation. Cynomolgus macaque monkeys given heart allografts were lymphodepleted using Alemtuzumab (Campath-1H; anti-CD52). Peripheral blood (PB) and lymph nodes (LN) were analyzed for CD95− (Tn) and CD95+ cells (Tmem), one day, one month and up to three months after Alemtuzumab infusion. CD52 expression, susceptibility to Alemtuzumab cytotoxicity and pro-apoptotic caspase-3 were evaluated in Tn and Tmem. In vivo, Alemtuzumab induction profoundly depleted lymphocytes in PB (99% reduction) but exerted a lesser effect in LN (70% reduction), with similar depletion of Tn and Tmem subsets. After transplantation, Tmem comprised the majority of lymphocytes in PB and LN. In vitro, LN T cells were more resistant to Alemtuzumabmediated cytotoxicity than PB lymphocytes. CD4+ Tn and Tmem were equally susceptible to Alemtuzumab-mediated cytotoxicity, whereas CD8+ Tn were more resistant than CD8+ Tmem. However, no significant differences in CD52 expression between lymphocyte subsets in PB and LN were observed. Caspase-3 expression was higher in PB than LN T cells. CD4+ and CD8+ Tn expressed lower levels of Caspase-3 than Tmem, in both PB and LN. Thus, after Alemtuzumab infusion, residual Tn in secondary lymphoid tissue may predispose to rapid recovery of Tmem in allograft recipients.
Keywords: alemtuzumab, transplantation, lymph node, memory T cells, naïve T cells, non-human primate
1. Introduction
Memory T cells (Tmem) exhibit several characteristics such as high levels (compared with naïve T cells; Tn) of adhesion molecule expression, less dependence on costimulatory signals and a distinct cytokine profile, that enhances their ability to clear foreign antigen [1–5]. These properties are a threat to graft survival after transplantation, where Tmem constitute a major barrier to tolerance induction [6, 7].
To overcome this problem, many agents have been used to induce lymphodepletion, such as Alemtuzumab and antithymocyte globulin (ATG) [8, 9]. Alemtuzumab (Campath-1H) is a humanized IgG1 monoclonal antibody (mAb) directed against CD52,- a cell surface glycoprotein expressed on all lymphocytes, natural killer cells, monocytes, and dendritic cells [10]. Alemtuzumab is used to treat leukemia [11], lymphomas [12], and autoimmune diseases such as rheumatoid arthritis [13]. It is also used as pre-operative induction therapy in transplantation due to its potent ability to deplete T and B cells from the circulation [8]. However, severe lymphodepletion following alemtuzumab infusion is associated with repopulation and rapid recovery of Tmem, that is also seen with other lymphodepleting agents [14–23], giving rise to the concept that Tmem are resistant to lymphodepletion. On the other hand, it has been reported that, in mice, Tmem repopulation after T cell depletional therapy results from homeostatic proliferation of naïve T cells (Tn) that possess a superior proliferative capacity in a lymphodepleted environment [24]. Moreover, it has been reported that naïve CD4 and CD8 T cells, recently emigrated from the thymus (CD31+ Tn), are the main contributors to early Tmem repopulation in both pediatric and adult populations despite thymic involution in adults [25]. In rodents, there is evidence that Tmem are the product of homeostatic proliferation of lymphodepletion-resistant Tmem and/or Tn [24]. Tmem repopulation in peripheral blood (PB) following lymphodepletion has been reported previously in both humans and non-human primates (NHP), but the mechanisms underlying Tmem repopulation are not fully understood [14–17, 19, 22, 26]. Moreover, little has been documented concerning the kinetics of lymphocyte depletion and recovery in lymphoid tissue of NHP or humans following T cell depletion. Here, we have performed a detailed, sequential analysis of the influence of alemtuzumab on depletion and recovery of lymphocyte subsets in the PB and lymph nodes (LN) of heart-transplanted Indonesian cynomolgus monkeys.
2. Materials and Methods
2.1 Animals
Healthy cynomolgus monkeys (Macaca fascicularis) of Indonesian origin, weighing 3–5kg, were obtained from specific pathogen-free colonies at Alpha Genesis, Inc, or the National Institute of Allergy and Infectious Diseases colony (both Yamassee, SC). All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86-23, revised 1985), and under a University of Pittsburgh Institutional Animal Care and Use Committee-approved protocol. Environmental enrichment was provided. Unlike humans, most NHP species express CD52 on both white and red blood cells, leading to severe anemia when using Alemtuzumab [27]. The Indonesian sub-species of cynomolgus macaque however, has been reported to be resistant to anemia induced by Alemtuzumab due to lack of CD52 expression on its erythrocytes [28, 29]. Cynomolgus monkey CD52 shares 85% structural homology with its human counterpart [30].
2.2 Immunosuppression and surgical procedures
Six monkeys received a heterotopic heart transplant from an ABO-compatible allogeneic donor on day 0 (Table 1). Anesthesia, heart excision in donor monkeys, and heterotopic intra-abdominal heart transplantation were performed as described [31]. On days −2 (two days before transplant), and on days 5 and 12 after transplant, the recipient was given an intravenous (i.v.) infusion of Alemtuzumab (Campath-1H; Genzyme, Cambridge, MA) at doses of 20, 10 and 10 mg/kg, respectively. Maintenance immunosuppression consisted of mycophenolate mofetil (MMF) (Genentech USA, Inc., South San Francisco, CA) from day -1 to 18 (target trough levels of 3–6 mg/mL), followed by rapamycin (LC Laboratories, Woburn, MA) from days 19 to 54 (target trough levels of 10–15 ng/mL) after which rapamycin was weaned slowly and discontinued completely on day 84.
Table 1.
Graft survival in Alemtuzumab-treated cynomolgus monkeys
| Monkey | Alemtuzumab (doses in mg/kg)a |
Maintenance therapy |
Graft survival (days) |
LN biopsy (days) |
|---|---|---|---|---|
| 51-11 | 20-10-10 | MMF/Rapa | 97 | 2, 84 |
| 54-11 | 20-10-10 | MMF/Rapa | 96 | 2, 84 |
| 212-11 | 20-10-10 | MMF/Rapa | 62 | 28, 56 |
| 213-11 | 20-10-10 | MMF/Rapa | 50 | 2, 28, 56 |
| 214-11 | 20-10-5 | MMF/Rapa | 39 | 2, 28, 56 |
| 216-11 | 10-5-5 | MMF/Rapa | 33 | 2, 28 |
First dose of alemtuzumab given 2d before transplant on day 0.
Lymph nodes (LN) were obtained from normal monkey donors or excised from four of the six graft recipient monkeys on d0 (on the day of transplant), 1, 2 or 3 months after transplant, and at euthanasia.
2.3 Collection and preparation of samples
Normal, untreated monkeys were used as blood and LN donors for in vitro experiments. Whole blood, Ficoll-purified PB mononuclear cells (PBMC) and LN were obtained from normal monkeys, either immediately upon isolation or after storage in liquid N2 (−80°C). Blood samples were drawn from the recipient monkeys before Alemtuzumab infusion, on day 0, then weekly after transplant to monitor T cell subsets. Calculation of absolute cell numbers was based on the WBC counts obtained from our Institution’s hematology laboratory and applying the % of positively stained cells by flow cytometric analysis.
LN obtained either from naïve or transplanted monkeys were weighed, and either stored in liquid N2 (−80°C), or used for cell isolation. Cells were isolated by gently mashing the tissue in a sterile petri dish. Lymphocytes were filtered through a 70µm cell strainer, washed with PBS, then counted to obtain cell numbers per mg LN, followed by staining and flow cytometric analysis.
2.4 Flow cytometric analysis
For cell surface staining, the following conjugated antibodies were used: PerCP-cy5 CD3 (clone: sp34-2), APC CD4 (clone: L200), APC-Cy7 CD8 (clone: RPA-T8), all from BD Pharmingen (San Diego, CA). CD95 PE-Cy7 (clone: DX2) from Biolegend (San Diego, CA). FITC CD52 (clone: YTH34.5) from Serotec (Raleigh, NC). FITC Caspase-3 (clone: C92–605) and Bcl-2 (clone: Bcl2/100) from Pharmingen BD. For intracellular staining, cell fixation and permeabilization were performed using Fix and Perm reagent (BD Pharmingen). Events were collected using an LSR-II (San Jose, CA), or Fortessa (BD Biosciences) flow cytometer, and analyzed with Flowjo software (Ashland, OR).
2.5 Alemtuzumab-mediated cytotoxicity assay
Stored PBMC (from PB), LN and autologous serum were obtained from the same normal monkeys (n=4). PBMC and LN cells were thawed and then 1× 106 cells were added to 5 ml flow tubes, washed with phosphate-buffered saline (PBS), and resuspended in RPMI media supplemented with 30% v/v autologous serum. Alemtuzumab was then added at different concentrations to appropriate flow tubes (10µg/ml, 50µg/ml, and 100µg/ml). Control tubes (no Alemtuzumab added) were included. All tubes were incubated at 37°C in a humidified atmosphere containing 5% CO2 for 4 hours. After incubation, the cells were washed with PBS, and then surface stained for flow analysis. CountBright absolute counting beads (Invitrogen) were added to each sample before collecting the flow events to count the cells based on the following formula (# of cell events / # of bead events = assigned bead count of the lot / volume of the sample).
2.6 Caspase-3 expression
To induce apoptosis, PBMC or LN cells were incubated with Camptothecin (Sigma; St. Louis, MO) in 6-well plates with each well containing 3 ml of RPMI + 10% fetal calf serum (FCS) media, Camptothecin (5 µM/well) and 4×106 cells. The cells were incubated for 8 hours at 37°C in a humidified atmosphere containing 5% CO2. After incubation, they were washed with PBS, surface stained, then fixed and permeabilized for intracellular staining.
2.7 Statistical analyses
Continuous variables were expressed as means ± standard deviation. Differences between groups were evaluated using Student’s paired ‘t’-test. Statistical analyses were conducted using the standard formulas in Microsoft Excel software; a ‘P’ value <0.05 was considered significant.
3. Results
3.1 Profound T cell depletion in PB but not in LN of heart transplant recipients after one dose of Alemtuzumab
An initial dose of Alemtuzumab resulted in nearly complete depletion of all T cells (97.5%±2%) in the PB (day 0 post-transplant; Figure 1). CD3+T cells declined from 3669±99/ml before Alemtuzumab to 99±74/ml (P<0.001), 2 days after its infusion. Alemtuzumab was less effective in LN, where it induced less T cell depletion (70%±11% in comparison to normal monkeys); CD3+T cells in LN 2 days after one dose were 234 ± 87 ×103/mg LN tissue, compared to normal monkeys, where CD3+ T cells in LN were 770 ± 290 ×103/mg LN tissue (P<0.001). One month after 3 doses of Alemtuzumab, the extent of depletion in LN increased to 85.5%±9%; CD3+ cells were 120 ± 76 ×103/mg LN tissue (p<0.001).
Fig. 1.
Total T cell (CD3+) depletion in peripheral blood (PB) and lymph nodes (LN) of cynomolgus macaques after Alemtuzumab infusion. Percentages of total CD3+ T cells are shown in PB (in comparison to pre- alemtuzumab infusion; n=6) and in LN (in comparison to normal monkeys; n=4) on day 0 (day of transplant; 2 days after Alemtuzumab) and 1 month post-transplant (after the third dose of Alemtuzumab). The pre-Alemtuzumab value in LN was estimated by using the average of CD3+ T cells in LN obtained from normal monkeys (n=6). (*** p<0.001)
3.2 Similar depletion of Tn and Tmem in the PB after Alemtuzumab infusion
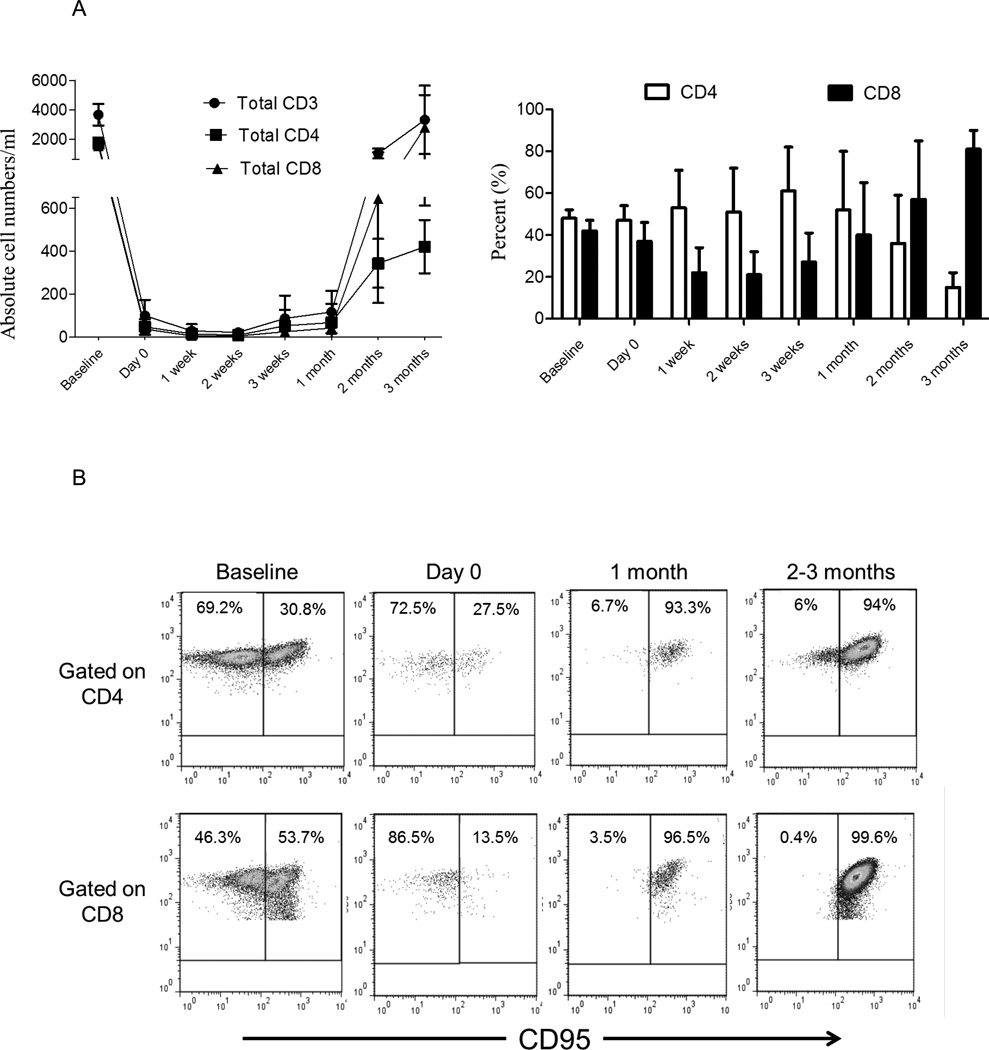
Total CD3+T cell numbers were suppressed efficiently by Alemtuzumab infusion. CD3+T cell numbers were maintained at < 20 ± 20 cells/ml, until 3 weeks post-transplant (1 week after the last dose of Alemtuzumab). During this period, CD8+T cell repopulation was more marked than that of CD4+T cells (Figure 2A), with higher percentages of CD8+ than CD4+T cells in the circulation. Based on cell surface staining, Tmem in NHP are considered CD95+, while Tn are CD95− [32]. We evaluated Tn and Tmem in the PB before and after Alemtuzumab infusion (Figure 2B). After the initial dose of Alemtuzumab (i.e. on day 0), there was similar, profound depletion of both Tn and Tmem in the PB (Figure 2B and C), for both CD4+T cells (97%±2% and 97%±1%, respectively) and CD8+T cells (93%±6% and 98%±1%, respectively). Following 2 doses of Alemtuzumab (i.e. 1 week after transplantation) and subsequently until the time of heart graft rejection between 1 and 3 months (Table 1), we observed that Tmem constituted the majority of T cells in the peripheral circulation (Figure 2C).
Fig. 2.
Naïve (Tn) and memory T cells (Tmem) in the peripheral blood (PB) before and after Alemtuzumab infusion. Blood samples were drawn from the heart graft recipient cynomolgus monkeys (n=6) before Alemtuzumab (baseline), on the day of transplant (day 0; 2 days after the first Alemtuzumab infusion), then weekly and monthly thereafter. After gating on CD3+CD4+ and CD3+CD8+ cells, Tn (CD95−) and Tmem (CD95+) subsets were enumerated. (A) Absolute numbers of CD3+, CD3+CD4+ and CD3+CD8+ T cells (left), and percentages (%) of total CD3+CD4+ and CD3+CD8+ T cells (right) in PB. (B) Flow cytometric analysis of Tn (CD95−) and Tmem (CD95+) in PB at various times, before and after Alemtuzumab infusion. (C) Absolute number and percentage (%) of baseline of CD4+ (upper) and CD8+ (lower) Tn (CD95−) and Tmem (CD95+) subsets, before and after Alemtuzumab infusion.
3.3 Similar depletion of Tn and Tmem in LN after Alemtuzumab infusion
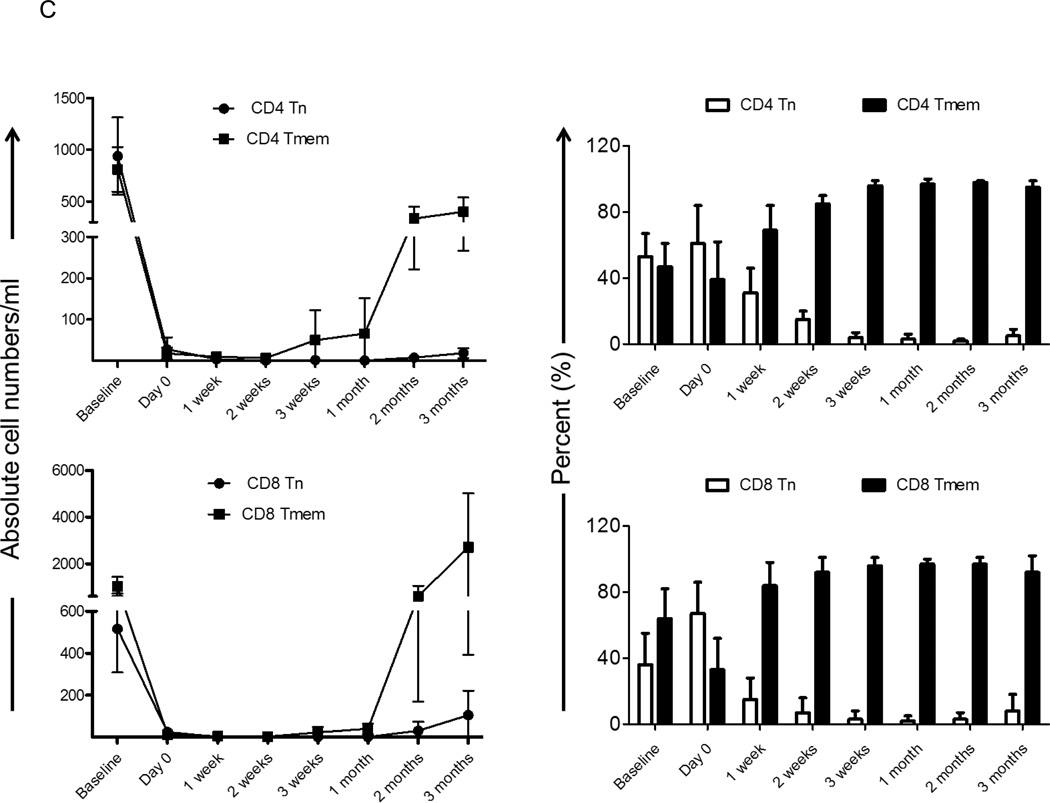
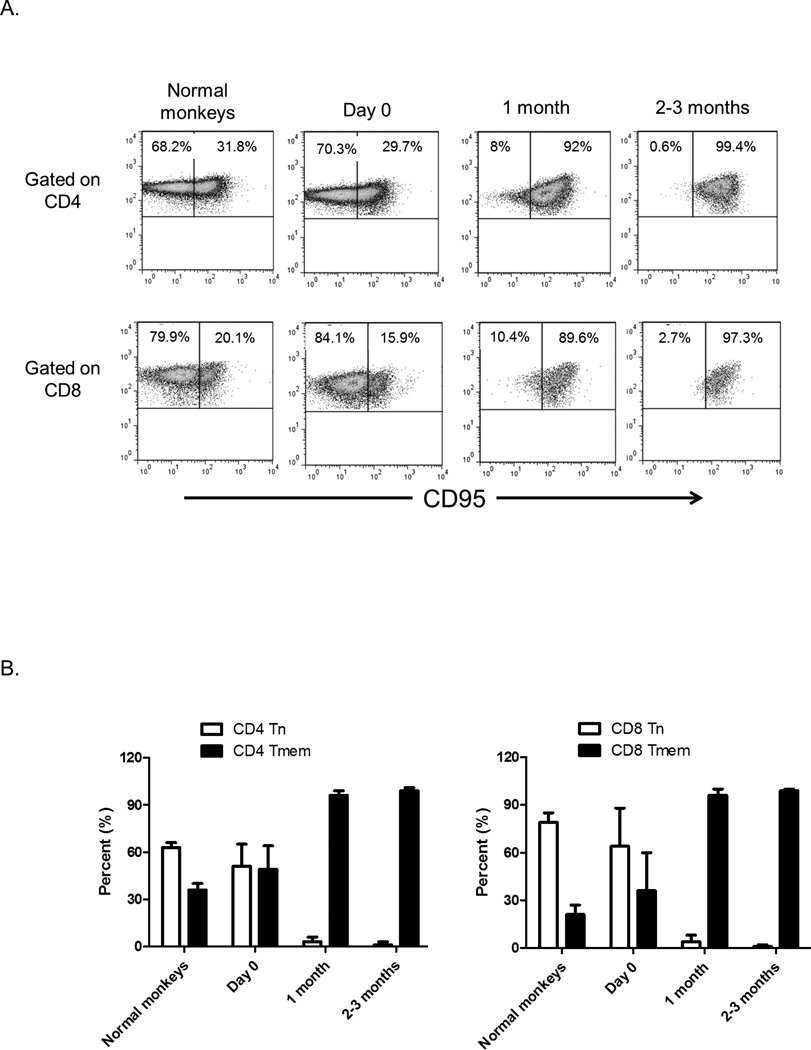
Because circulating lymphocytes in the periphery at any given time represent only a small percentage of total lymphocytes, we examined lymphocyte depletion in the secondary lymphoid organs to provide a more accurate picture of T cell depletion. Based on CD95 expression, we evaluated Tn and Tmem in LN CD4+ and CD8+T cell populations following Alemtuzumab infusion in the monkey heart transplant recipients and compared the data to LN obtained from normal monkeys (Figure 3A). As shown in Figure 1, T cell depletion was evident in LN after the first dose of Alemtuzumab (day 0, although the effect was not as marked as in PB). Following the first dose of Alemtuzumab, the percentages of Tn and Tmem for both CD4+T cells (51%±14% and 49%±15%, respectively) and CD8+T cells (64%±24% and 36%±24%, respectively) were similar to those in normal monkeys (Figures 3A and 3B), indicating that Tn and Tmem in LN was depleted to similar extents. However, at 1 month, Tmem constituted the majority of T cells in LN. The incidence of CD4 Tn was 3%±3%, while that of CD4 Tmem was 97%±3%. Similarly, CD8 Tn represented 4%±4%, while CD8 Tmem represented 96%±4%. At 2–3 months, the percentages of Tn and Tmem remained similar to those at 1 month.
Fig. 3.
Repopulation of cynomolgus monkey T cells in lymph nodes (LN) after Alemtuzumab infusion. (A) Tn and Tmem CD4+ and CD8+ T cell populations were identified in LN based on differential CD95 expression. Tn (CD95−) and Tmem (CD95+) from a normal monkey, and a representative heart transplant recipient on the day of transplant (day 0; 2 days after Alemtuzumab), at one month and at 2–3 months after transplantation are shown. (B) Percentages of CD4+ and CD8+ Tn and Tmem in normal monkeys (n=6) and heart transplant recipients (n=4). Two days after Alemtuzumab infusion, similar proportions of Tn and Tmem in both CD4+ and CD8+ T cell populations were observed. However, at one month and 2–3 months, the vast majority of CD4+ and CD8+ T cells in LN were Tmem.
3.4 CD4 naïve and memory T cells are equally susceptible, while CD8 naïve T cells are relatively resistant to Alemtuzumab-mediated depletion in vitro
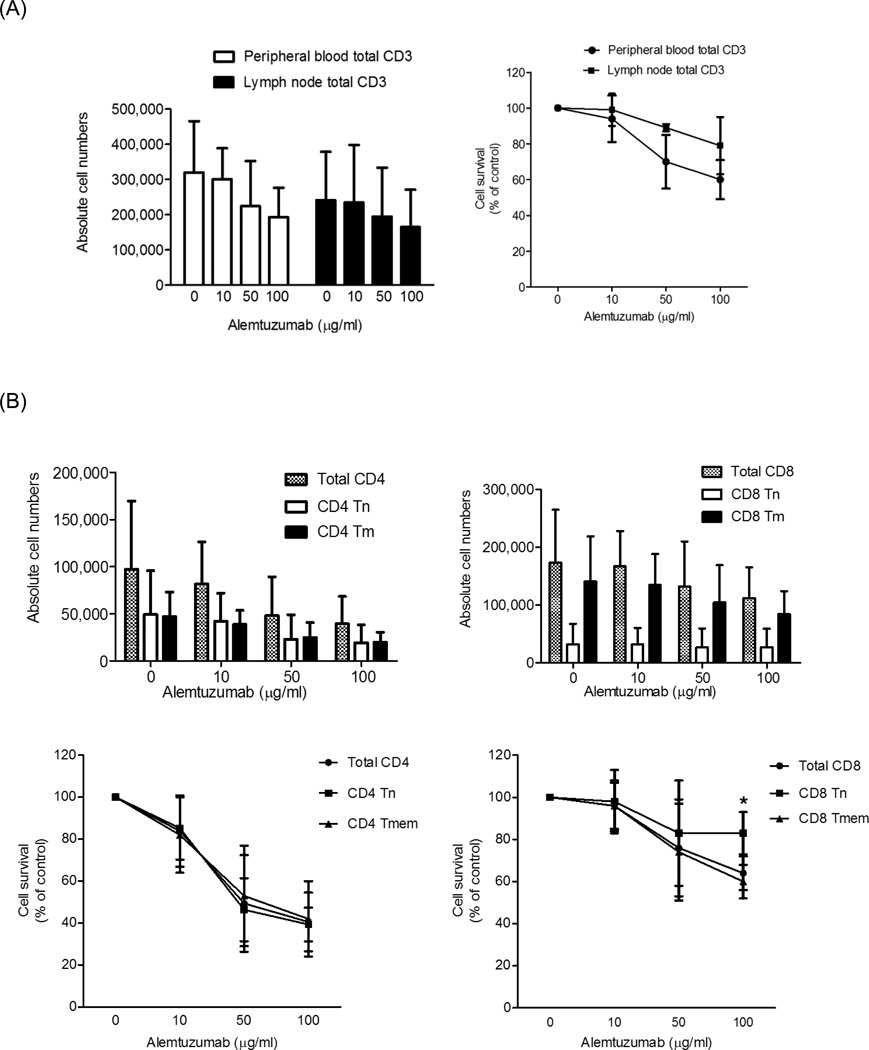
At 1 month after Alemtuzumab, Tmem (particularly CD8+) constituted the major T cell population in the peripheral circulation and LN of the heart graft recipients. We considered that this might be due to a disparate depleting effect of Alemtuzumab on Tmem versus Tn. Thus we evaluated the influence of Alemtuzumab on Tn and Tmem in cytotoxicity assays in vitro (Figure 4). Targets were lymphocytes isolated from normal cynomolgus monkey LN, and PBMC isolated from PB. Target cells were incubated with autologous serum and different concentrations of Alemtuzumab for 4 hours. Percentages and absolute numbers of CD3+, CD4+ and CD8+T cell populations were calculated by flow cytometry.
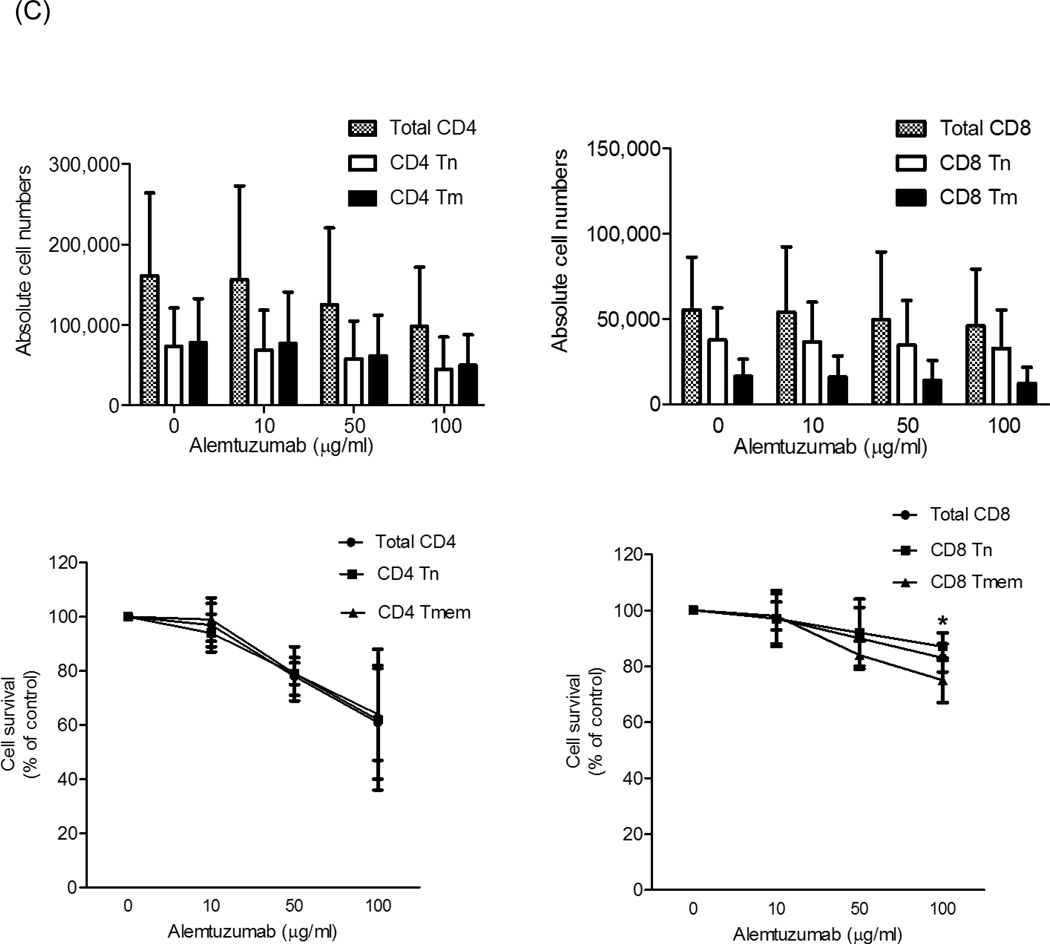
Fig. 4.
Alemtuzumab-mediated cytotoxicity in vitro. Cynomolgus monkey peripheral blood (PB) or lymph node (LN) cells were incubated for 4 hours with alemtuzumab at various concentrations (10, 50, and 100µg/ml), together with autologous serum, then analyzed by flow cytometry. Absolute cell numbers were calculated using CountBright beads. Cell survival determination was based on the absolute number of cells at each concentration compared to controls (no Alemtuzumab). (A) Absolute numbers of CD3+T cells (left) and the percent surviving cells (right) from PB or LN. (B) Upper panels show the absolute numbers of CD4+ and CD8+T cells; lower panels show the percent surviving cells from PB. (C) Absolute numbers (upper panels) and percent survival (lower panels) for CD4+ (left) and CD8+ (right) LN T cells. For both PB and LN, CD8+ Tn were significantly more resistant to Alemtuzumab-mediated cytotoxicity than CD8+ Tmem; *p<0.01. Data are representative of 4 different experiments.
As seen in Figure 4A, Alemtuzumab killed total CD3+T cells from both PB and LN. At 100µg/ml, PB CD3+T cell survival was 60%±11%, while that of LN CD3+T cells was 79%±16%. At 100µg/ml concentration, Alemtuzumab-induced cytotoxicity was evident in PB total CD4+ and CD8+T cell populations (Figure 4B). PB CD4+T cells survival was 41%±14%, while CD8+T cell survival was 64%±8%. Among CD4+T cells, the effect of Alemtuzumab on both Tn and Tmem was similar (survival at 100µg/ml was 39%±8% and 42%±18%, respectively) (Figure 4B). For PB CD8+T cells, Tn were significantly more resistant to killing by Alemtuzumab than Tmem (survival at 100µg/ml was 83%±10% and 60%±8%, respectively; p<0.01) (Figure 4B).
For LN lymphocytes, at 100 µg/ml, Alemtuzumab-induced cytotoxicity was evident in total CD4+ and CD8+T cells populations, as for PB cells. LN CD4+T cell survival was 61%±21%, while CD8+T cell survival was 83%±5% (Figure 4C). Among CD4+T cells, the effect of Alemtuzumab on both Tn and Tmem was similar (survival at 100µg/ml was 62%±26% and 64%±17%, respectively). For LN CD8+T cells, Tn were also significantly more resistant than Tmem (survival at 100µg/ml was 87%±5% and 75%±8%, respectively; p<0.01). These data indicate that cynomolgus monkey PB and LN CD8+T cells are significantly more resistant to Alemtuzumab killing than CD4+T cells (p<0.05), with CD8+ Tn cells being the most resistant.
3.5 CD52 expression on T cells in LN and PB
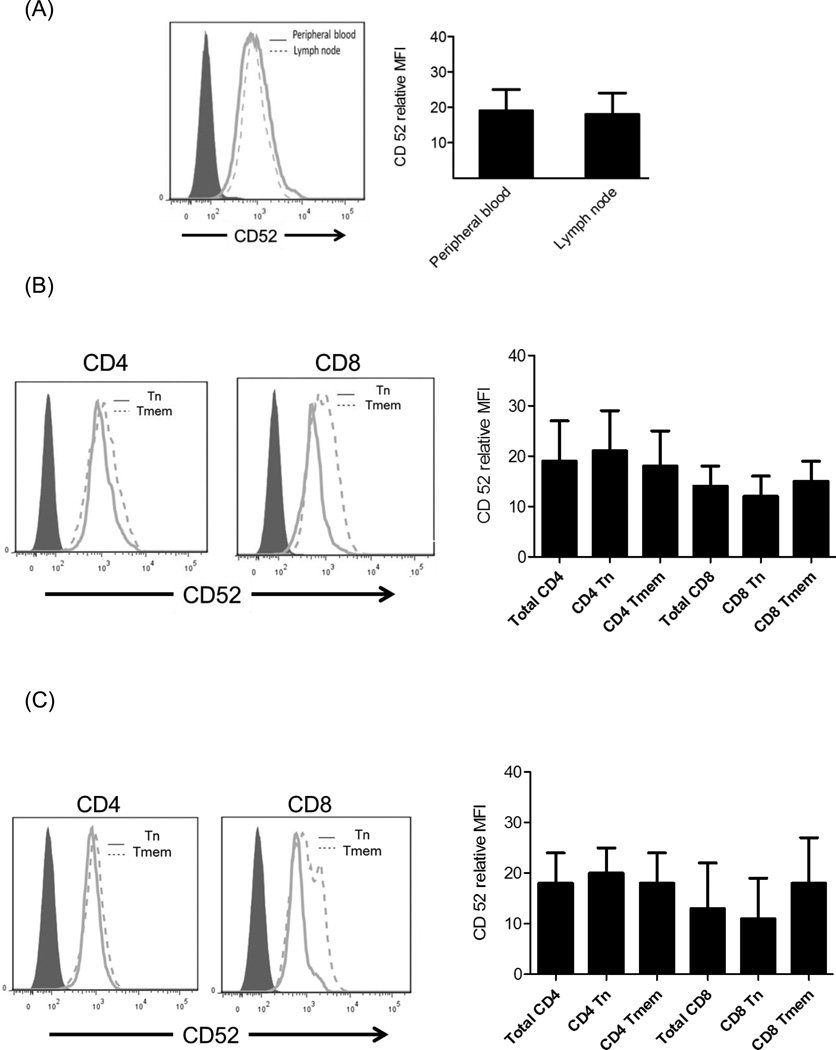
The differences observed in susceptibility to Alemtuzumab-mediated cytotoxicity may be attributable to differences in the binding of Alemtuzumab to lymphocyte sub-populations. Thus, we evaluated the expression of CD52 on different T cell subsets in both PB and LN using flow cytometry. As seen in Figure 5A, CD52 expression on total CD3+T cells in PB was similar to that on CD3+T cells in LN (relative MFI: 18±6 and 19±6, respectively). Although there was a trend towards higher CD52 expression on CD4+ than CD8+ T cells, there was no significant difference in CD52 expression between PB and LN. Interestingly, the lowest CD52 expression was observed on CD8 Tn.
Fig. 5.
CD52 expression on cynomolgus monkey lymphocyte subsets. CD52 expression on (A) CD3+T cells, (B) CD4+ and CD8+T cells in PB and (C) LN of normal monkeys evaluated by flow cytometry (n=4). Histograms on the left are from representative animals. Graphs on the right show means of 4 different normal monkeys.
3.6 T cell sub-populations in LN express less Caspase-3 than those in PB
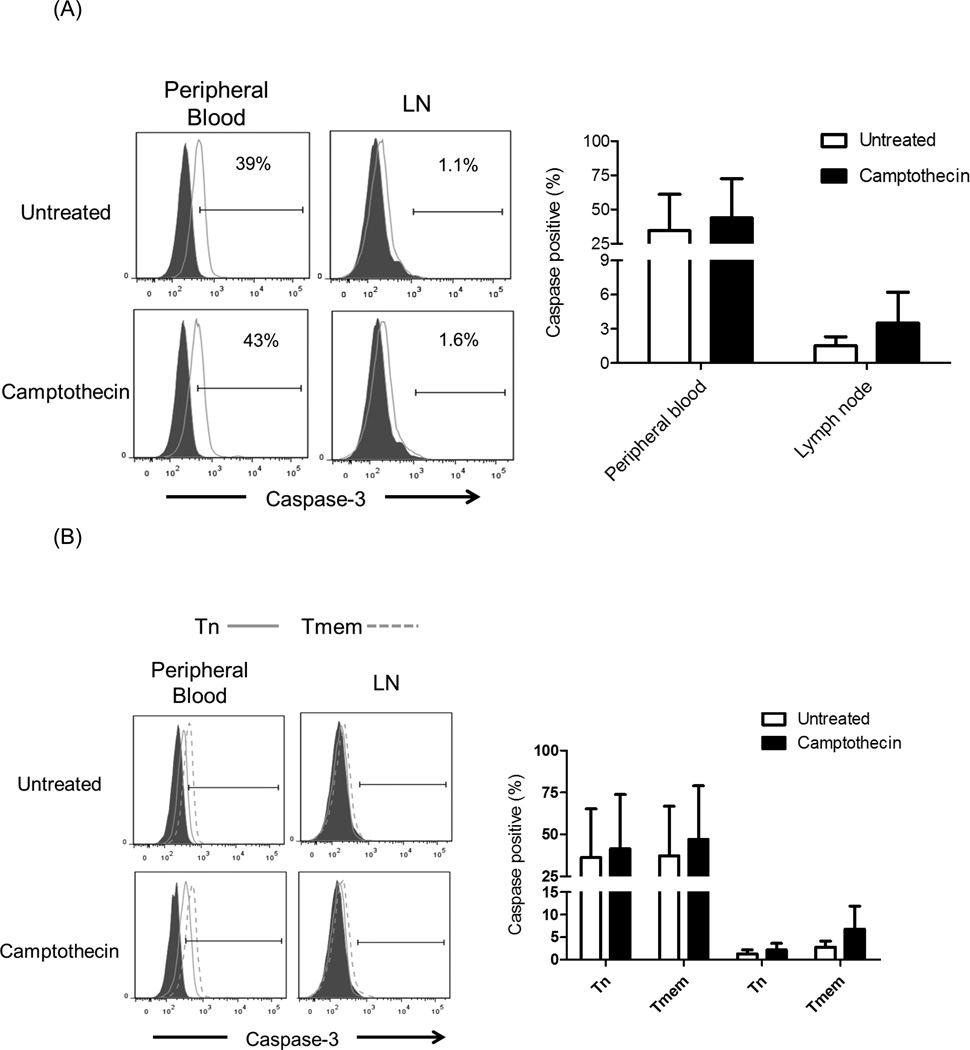
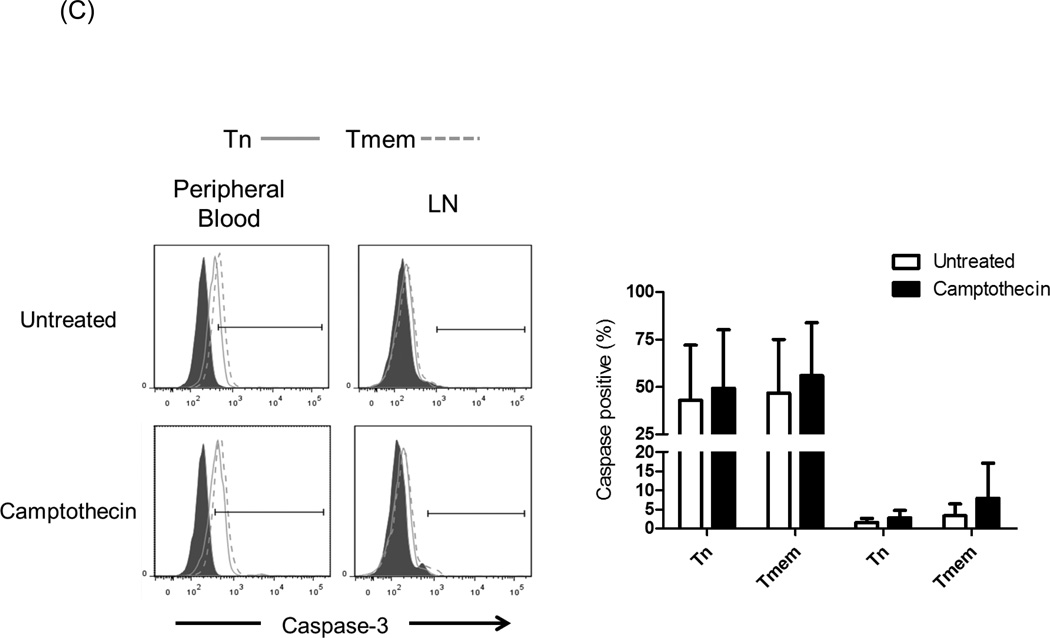
Resistance to apoptosis may play a role in the susceptibility of T cell subsets in PB and LN to Alemtuzumab-induced killing. Cells from PB and LN were evaluated for apoptosis induction and the expression of anti-apoptotic (Bcl-2) [33] and pro-apoptotic (caspase-3) [34] molecules. To promote apoptosis, the cells were incubated with 5µM Camptothecin [35] for 8 hours. After this period, there was no difference in the expression of Bcl-2 by different T cell subsets (data not shown). As seen in Figure 6, caspase-3 expression was significantly higher in all subsets in PB, compared with LN T cells (p<0.05). There were increased percentages of caspase-3+ cells after Camptothecin exposure for all subsets. Moreover, there were more caspase-3+ cells among Tmem than Tn, suggesting that Tn are more resistant to apoptosis induction. There were no significant differences in caspase-3 expression between CD4+ and CD8+T cells before or after camptothecin treatment. Collectively, these data suggest that combined lower expression of caspase-3 and CD52 by CD8+ Tn may promote resistance to Alemtuzumab-mediated killing in vitro.
Fig. 6.
Caspase-3 expression. Cynomolgus monkey peripheral blood (PB) and lymph node (LN) cells were incubated with 5µM Camptothecin for 8 hours. Control samples were untreated cells. Following culture, the cells were stained for intracellular expression of Caspase-3 by flow cytometry. Caspase-3 expression in (A) CD3+T cells, (B) CD4+T cells, and (C) CD8+T cells was evaluated. Histograms (left) are from one representative monkey from each group; grey histograms indicate isotype controls. Graphs (right) represent mean values obtained from normal monkeys (n=4).
4. Discussion
We evaluated T cell repopulation following lymphodepletion by Alemtuzumab in heart-transplanted cynomolgus monkeys. It has been reported that, in humans, depletion in the peripheral circulation occurs as early as one hour after Alemtuzumab infusion [14]. Our data show that a single dose of Alemtuzumab leads to profound depletion of Tn and Tmem in the PB (97.5%), but not to the same extent (70%) in LN within 24 hours. After one dose of Alemtuzumab, only 70% depletion of total CD3+T cells occurred in the LN, with similar depletion of both Tn and Tmem populations. Kirk et al [14] have reported that human iliac LN taken after three doses of Alemtuzumab exhibit predominance of a CD45RO+ Tmem population. This is consistent with our current data obtained in monkeys after a third dose of Alemtuzumab, were Tmem represented >96% of both CD4+ and CD8+T cells.
Repopulation of T cells occurred by 3–4 weeks post-transplant, as reported previously for ATG in NHP [18] and Alemtuzumab in humans [17]. In the present study, CD8+T cells reached pretreatment levels by 3 months post-transplant, while CD4+T cells were < 50% of pre-treatment levels at this time. In humans, T cells recover to 50% of pretreatment levels by 36 months, with initial recovery of CD8+ T cells, followed by CD4+T cells at later time points [17]. In normal cynomolgus monkeys given Alemtuzumab in the absence of transplant, complete CD8+T cell recovery occurred by day 35, while CD4+T cell recovery occurred by day 56 [36]. While our observations are consistent with those of others [20, 24, 29, 37], some reports indicate that Tmem are more resistant to depletion [16, 19]. In these latter reports, depletion by Alemtuzumab has not been studied after a single infusion. It is known however, that lymphocyte sub-populations in the LN differ from those in the PB in their activation status and survival [9, 38–40].
To further evaluate the influence of Alemtuzumab on Tn versus Tmem, we performed cytotoxicity assays as described [41]. In correlation with our in vivo observations, CD3+T cells in LN were more resistant to killing than CD3+T cells in PB. Also, CD4+T cells were more susceptible to Alemtuzumab-mediated killing than CD8+T cells in PB, as reported previously [41]. While CD4+ Tn and Tmem were equally depleted, CD8+ Tn were more resistant to depletion than Tmem. These observations contradict previously reported human data [16], where Tmem were more resistant to in vitro depletion. Others have shown that CD4+ and CD8+T cell subsets are depleted equally by Alemtuzumab in vitro [42]. This discrepancy may be due to the different species studied (human versus NHP), and to use of different cytotoxicity assays. Of note, our analyses were based on distinct phenotypes for Tn (CD95−) and Tmem (C95+) in NHP and did not include further subtypes of Tmem (effector memory and central memory) based on CD28 expression.
In vivo T cell resistance to Alemtuzumab depletion in LN may be due to differences in the bioavailability of Alemtuzumab in LN and PB, where more than one dose of Alemtuzumab can induce more T cell depletion (Figure1). Another possible explanation is variable binding of Alemtuzumab to lymphocytes in PB versus LN. Although we found no significant differences in CD52 expression between populations, it was least expressed on CD8+ Tn. Our data suggest that inadequate depletion in LN after Alemtuzumab infusion may provide a reservoir of T cells that can contribute to Tmem repopulation, as reported previously in mice and NHP [37, 43]. Our observations also suggest that repopulation of Tmem following their depletion in monkey heart transplant recipients may be due either to expansion of remaining Tmem that survive initial depletion by Alemtuzumab [44], or to homeostatic expansion of Tn [24, 25] and their conversion of to Tmem after transplantation [45–47]. Studies in mice [48] have shown that Tn undergoing homeostasis-driven proliferation can convert to a phenotypic and functional state similar to that of Tmem, but distinct from Ag-activated effector T cells. These ‘pseudo-memory’ T cells revert to a naïve phenotype once the cellularity of the lymphoid compartment is restored. It is not clear whether these cells participate in rejection and/or resistance to tolerance. It would have been of interest to ascertain (e.g. using a MLR assay) the frequency of both alloreactive and donor-specific T cells amongst the T cells that underwent homeostatic expansion in our model. However, the very limited numbers of lymphocytes that could be isolated from blood or LN following profound lymphodepletion precluded these functional analyses.
A recent report by Nadazdin et al [49] suggests that expansion of donor-specific Tmem rather than overall homeostatic expansion of Tmem is relevant to rejection in renal-allografted NHP (cynomolgus monkeys). Thus ‘true’ long-lived Tmem (as opposed to ‘pseudo-memory’ T cells) are characterized by high levels of anti-apoptotic gene expression. Differences in the expression of proand anti-apoptotic molecules among Tn and Tmem have been reported [50–52]. Here, we investigated the expression of Bcl-2 in Tn and Tmem, but found no difference between different T cell subsets in both blood and LN, either before or after apoptosis induction with Camptothecin (data not shown). This correlates with previously reported data showing that Bcl-2 plays only a minor role in Alemtuzumab-induced apoptosis [53]. We have found increased pro-apoptotic caspase-3 expression in Tmem cells in both PB and LN. These data are consistent with previously reported evidence that the T central memory population has higher caspase-3 levels [52], while others have reported that CD8+ effector and terminally-differentiated effector memory sub-populations have less caspase 3 when compared to naïve and central memory T cells [54]. In our cynomolgus monkey model, resistance to apoptosis of LN cells in comparison to PB cells correlates with resistance to Alemtuzumab-mediated cytotoxicity. This may be relevant to lower levels of depletion of LN cells after Alemtuzumab in vivo.
5. Conclusion
In conclusion, our data extend previous observations regarding repopulation of Tmem after lymphocyte depletion in organ allograft recipients. They also provide further insight into factors that contribute to Tmem survival and recovery after organ allotransplantation. Ineffective depletion of LN T cells and relative resistance of Tn (particularly CD8+ Tn) to Alemtuzumab killing is likely to contribute to the preferential repopulation of Tmem through the conversion of Tn to Tmem after transplantation.
Highlights.
Alemtuzumab induction depletes peripheral blood more than LN lymphocytes
After transplantation, memory T cells comprise the majority of lymphocytes
LN T cells are more resistant to Alemtuzumab-mediated killing than blood T cells
After depletion, naïve T cells in LN may account for rapid recovery of memory T cells
Acknowledgments
The authors’ work is supported by National Institutes of Health grants U01 AI091197 (AWT and DKCC), U01 AI51698 and R01 AI67541 (AWT) and by the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh. Eefje M. Dons was in receipt of fellowships from the Ter Meulen Fund of the Royal Netherlands Academy of Arts and Sciences and the Stichting Professor Michael van Vloten Fund, The Netherlands. Mohamed Ezzelarab was supported in part by the Shelly Patrick Fellowship of the Thomas E. Starzl Transplantation Institute.
Abbreviations
- ATG
anti-thymocyte globulin
- LN
lymph node(s)
- MFI
mean fluorescence intensity
- NHP
non-human primate
- PBMC
peripheral blood mononuclear cells
- Tmem
memory T cell(s)
- Tn
naïve T cell(s)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors report no conflicts of interest.
References
- 1.Bachmann MF, Barner M, Viola A, Kopf M. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur J Immunol. 1999;29:291–299. doi: 10.1002/(SICI)1521-4141(199901)29:01<291::AID-IMMU291>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 2.Budd RC, Cerottini JC, Horvath C, Bron C, Pedrazzini T, Howe RC, et al. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J Immunol. 1987;138:3120–3129. [PubMed] [Google Scholar]
- 3.Damle NK, Klussman K, Linsley PS, Aruffo A. Differential costimulatory effects of adhesion molecules B7, ICAM-1, LFA-3, and VCAM-1 on resting and antigen-primed CD4+ T lymphocytes. J Immunol. 1992;148:1985–1992. [PubMed] [Google Scholar]
- 4.Rogers PR, Dubey C, Swain SL. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J Immunol. 2000;164:2338–2346. doi: 10.4049/jimmunol.164.5.2338. [DOI] [PubMed] [Google Scholar]
- 5.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 6.Ford ML, Larsen CP. Overcoming the memory barrier in tolerance induction: molecular mimicry and functional heterogeneity among pathogen-specific T-cell populations. Curr Opin Organ Transplant. 2010;15:405–410. doi: 10.1097/MOT.0b013e32833b7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valujskikh A. The challenge of inhibiting alloreactive T-cell memory. Am J Transplant. 2006;6:647–651. doi: 10.1111/j.1600-6143.2005.01215.x. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal A, Shen LY, Kirk AD. The role of alemtuzumab in facilitating maintenance immunosuppression minimization following solid organ transplantation. Transpl Immunol. 2008;20:6–11. doi: 10.1016/j.trim.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21:1387–1394. doi: 10.1038/sj.leu.2404683. [DOI] [PubMed] [Google Scholar]
- 10.Hale G, Xia MQ, Tighe HP, Dyer MJ, Waldmann H. The CAMPATH-1 antigen (CDw52) Tissue Antigens. 1990;35:118–127. doi: 10.1111/j.1399-0039.1990.tb01767.x. [DOI] [PubMed] [Google Scholar]
- 11.Osterborg A, Dyer MJ, Bunjes D, Pangalis GA, Bastion Y, Catovsky D, et al. Phase II multicenter study of human CD52 antibody in previously treated chronic lymphocytic leukemia. European Study Group of CAMPATH-1H Treatment in Chronic Lymphocytic Leukemia. J Clin Oncol. 1997;15:1567–1574. doi: 10.1200/JCO.1997.15.4.1567. [DOI] [PubMed] [Google Scholar]
- 12.Lundin J, Osterborg A, Brittinger G, Crowther D, Dombret H, Engert A, et al. CAMPATH-1H monoclonal antibody in therapy for previously treated low-grade non-Hodgkin's lymphomas: a phase II multicenter study. European Study Group of CAMPATH-1H Treatment in Low-Grade Non-Hodgkin's Lymphoma. J Clin Oncol. 1998;16:3257–3263. doi: 10.1200/JCO.1998.16.10.3257. [DOI] [PubMed] [Google Scholar]
- 13.Isaacs JD, Watts RA, Hazleman BL, Hale G, Keogan MT, Cobbold SP, et al. Humanised monoclonal antibody therapy for rheumatoid arthritis. Lancet. 1992;340:748–752. doi: 10.1016/0140-6736(92)92294-p. [DOI] [PubMed] [Google Scholar]
- 14.Kirk AD, Hale DA, Mannon RB, Kleiner DE, Hoffmann SC, Kampen RL, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003;76:120–129. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 15.Kirk AD, Mannon RB, Kleiner DE, Swanson JS, Kampen RL, Cendales LK, et al. Results from a human renal allograft tolerance trial evaluating T-cell depletion with alemtuzumab combined with deoxyspergualin. Transplantation. 2005;80:1051–1059. doi: 10.1097/01.tp.0000174341.49741.8f. [DOI] [PubMed] [Google Scholar]
- 16.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 17.Bloom DD, Hu H, Fechner JH, Knechtle SJ. T-lymphocyte alloresponses of Campath-1H-treated kidney transplant patients. Transplantation. 2006;81:81–87. doi: 10.1097/01.tp.0000191940.13473.59. [DOI] [PubMed] [Google Scholar]
- 18.Haanstra KG, Sick EA, Ringers J, Wubben JA, Kuhn EM, t Hart BA, et al. No synergy between ATG induction and costimulation blockade induced kidney allograft survival in rhesus monkeys. Transplantation. 2006;82:1194–1201. doi: 10.1097/01.tp.0000235910.47214.67. [DOI] [PubMed] [Google Scholar]
- 19.Trzonkowski P, Zilvetti M, Friend P, Wood KJ. Recipient memory-like lymphocytes remain unresponsive to graft antigens after CAMPATH-1H induction with reduced maintenance immunosuppression. Transplantation. 2006;82:1342–1351. doi: 10.1097/01.tp.0000239268.64408.84. [DOI] [PubMed] [Google Scholar]
- 20.Louis S, Audrain M, Cantarovich D, Schaffrath B, Hofmann K, Janssen U, et al. Long-term cell monitoring of kidney recipients after an antilymphocyte globulin induction with and without steroids. Transplantation. 2007;83:712–721. doi: 10.1097/01.tp.0000255683.66156.d3. [DOI] [PubMed] [Google Scholar]
- 21.Serban G, Whittaker V, Fan J, Liu Z, Manga K, Khan M, et al. Significance of immune cell function monitoring in renal transplantation after Thymoglobulin induction therapy. Hum Immunol. 2009;70:882–890. doi: 10.1016/j.humimm.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 22.Toso C, Edgar R, Pawlick R, Emamaullee J, Merani S, Dinyari P, et al. Effect of different induction strategies on effector, regulatory and memory lymphocyte sub-populations in clinical islet transplantation. Transpl Int. 2009;22:182–191. doi: 10.1111/j.1432-2277.2008.00746.x. [DOI] [PubMed] [Google Scholar]
- 23.Cherkassky L, Lanning M, Lalli PN, Czerr J, Siegel H, Danziger-Isakov L, et al. Evaluation of alloreactivity in kidney transplant recipients treated with antithymocyte globulin versus IL-2 receptor blocker. Am J Transplant. 2011;11:1388–1396. doi: 10.1111/j.1600-6143.2011.03540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sener A, Tang AL, Farber DL. Memory T-cell predominance following T-cell depletional therapy derives from homeostatic expansion of naive T cells. Am J Transplant. 2009;9:2615–2623. doi: 10.1111/j.1600-6143.2009.02820.x. [DOI] [PubMed] [Google Scholar]
- 25.Gurkan S, Luan Y, Dhillon N, Allam SR, Montague T, Bromberg JS, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant. 2010;10:2132–2141. doi: 10.1111/j.1600-6143.2010.03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macedo C, Walters JT, Orkis EA, Isse K, Elinoff BD, Fedorek SP, et al. Long-term effects of alemtuzumab on regulatory and memory T-cell subsets in kidney transplantation. Transplantation. 2012;93:813–821. doi: 10.1097/TP.0b013e318247a717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hale G, Hoang T, Prospero T, Watt SM, Waldmann H. Removal of T cells from bone marrow for transplantation. Comparison of rat monoclonal anti-lymphocyte antibodies of different isotypes. Mol Biol Med. 1983;1:305–319. [PubMed] [Google Scholar]
- 28.Hale G, Swirsky DM, Hayhoe FG, Waldmann H. Effects of monoclonal anti-lymphocyte antibodies in vivo in monkeys and humans. Mol Biol Med. 1983;1:321–334. [PubMed] [Google Scholar]
- 29.van der Windt DJ, Smetanka C, Macedo C, He J, Lakomy R, Bottino R, et al. Investigation of lymphocyte depletion and repopulation using alemtuzumab (Campath-1H) in cynomolgus monkeys. Am J Transplant. 2010;10:773–783. doi: 10.1111/j.1600-6143.2010.03050.x. [DOI] [PubMed] [Google Scholar]
- 30.Yeung CH, Schroter S, Wagenfeld A, Kirchhoff C, Kliesch S, Poser D, et al. Interaction of the human epididymal protein CD52 (HE5) with epididymal spermatozoa from men and cynomolgus monkeys. Mol Reprod Dev. 1997;48:267–275. doi: 10.1002/(SICI)1098-2795(199710)48:2<267::AID-MRD15>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 31.Cooper DK, Ye Y, Niekrasz M. heart transplantation in primates. In: Cramer DV, Podesta L, Makowka L, editors. Handbook of Animal Models in Transplantation Research. Boca Raton, FL: CRC Press, Inc; 1994. pp. 173–200. [Google Scholar]
- 32.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 33.Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 34.Turnbull KJ, Brown BL, Dobson PR. Caspase-3-like activity is necessary but not sufficient for daunorubicin-induced apoptosis in Jurkat human lymphoblastic leukemia cells. Leukemia. 1999;13:1056–1061. doi: 10.1038/sj.leu.2401438. [DOI] [PubMed] [Google Scholar]
- 35.Bruno S, Lassota P, Giaretti W, Darzynkiewicz Z. Apoptosis of rat thymocytes triggered by prednisolone, camptothecin, or teniposide is selective to G0 cells and is prevented by inhibitors of proteases. Oncol Res. 1992;4:29–35. [PubMed] [Google Scholar]
- 36.Shan T, Qu L, Zhang J, Li Q, Shen B, Gu L, et al. Lymphocyte depletion and repopulation in peripheral blood and small intestine of cynomolgus monkeys after alemtuzumab treatment. J Surg Res. 2011;167:e21–e27. doi: 10.1016/j.jss.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 37.Page EK, Page AJ, Kwun J, Gibby AC, Leopardi F, Jenkins JB, et al. Enhanced de novo alloantibody and antibody-mediated injury in rhesus macaques. Am J Transplant. 2012;12:2395–2405. doi: 10.1111/j.1600-6143.2012.04074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Battaglia A, Ferrandina G, Buzzonetti A, Malinconico P, Legge F, Salutari V, et al. Lymphocyte populations in human lymph nodes. Alterations in CD4+ CD25+ T regulatory cell phenotype and T-cell receptor Vbeta repertoire. Immunology. 2003;110:304–312. doi: 10.1046/j.1365-2567.2003.01742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Genestier L, Fournel S, Flacher M, Assossou O, Revillard JP, Bonnefoy-Berard N. Induction of Fas (Apo-1, CD95)-mediated apoptosis of activated lymphocytes by polyclonal antithymocyte globulins. Blood. 1998;91:2360–2368. [PubMed] [Google Scholar]
- 40.Santin AD, Ravaggi A, Bellone S, Pecorelli S, Cannon M, Parham GP, et al. Tumor-infiltrating lymphocytes contain higher numbers of type 1 cytokine expressors and DR+ T cells compared with lymphocytes from tumor draining lymph nodes and peripheral blood in patients with cancer of the uterine cervix. Gynecol Oncol. 2001;81:424–432. doi: 10.1006/gyno.2001.6200. [DOI] [PubMed] [Google Scholar]
- 41.Lowenstein H, Shah A, Chant A, Khan A. Different mechanisms of Campath-1H-mediated depletion for CD4 and CD8 T cells in peripheral blood. Transpl Int. 2006;19:927–936. doi: 10.1111/j.1432-2277.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 42.Rao SP, Sancho J, Campos-Rivera J, Boutin PM, Severy PB, Weeden T, et al. Human peripheral blood mononuclear cells exhibit heterogeneous CD52 expression levels and show differential sensitivity to alemtuzumab mediated cytolysis. PLoS One. 2012;7:e39416. doi: 10.1371/journal.pone.0039416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu Y, Turner MJ, Shields J, Gale MS, Hutto E, Roberts BL, et al. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology. 2009;128:260–270. doi: 10.1111/j.1365-2567.2009.03115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ayasoufi K, Yu H, Fan R, Wang X, Williams J, Valujskikh A. Pretransplant antithymocyte globulin has increased efficacy in controlling donor-reactive memory T cells in mice. Am J Transplant. 2013;13:589–599. doi: 10.1111/ajt.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ge Q, Hu H, Eisen HN, Chen J. Different contributions of thymopoiesis and homeostasis-driven proliferation to the reconstitution of naive and memory T cell compartments. Proc Natl Acad Sci U S A. 2002;99:2989–2994. doi: 10.1073/pnas.052714099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mackall CL, Bare CV, Granger LA, Sharrow SO, Titus JA, Gress RE. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol. 1996;156:4609–4616. [PubMed] [Google Scholar]
- 47.Tanchot C, Le Campion A, Martin B, Leaument S, Dautigny N, Lucas B. Conversion of naive T cells to a memory-like phenotype in lymphopenic hosts is not related to a homeostatic mechanism that fills the peripheral naive T cell pool. J Immunol. 2002;168:5042–5046. doi: 10.4049/jimmunol.168.10.5042. [DOI] [PubMed] [Google Scholar]
- 48.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nadazdin O, Boskovic S, Murakami T, Tocco G, Smith RN, Colvin RB, et al. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci Transl Med. 2011;3:86ra51. doi: 10.1126/scitranslmed.3002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta S, Agrawal S, Gollapudi S. Differential effect of human herpesvirus 6A on cell division and apoptosis among naive and central and effector memory CD4+ and CD8+ T-cell subsets. J Virol. 2009;83:5442–5450. doi: 10.1128/JVI.00106-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta S, Gollapudi S. Susceptibility of naive and subsets of memory T cells to apoptosis via multiple signaling pathways. Autoimmun Rev. 2007;6:476–481. doi: 10.1016/j.autrev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 52.Gupta S, Gollapudi S. CD95-mediated apoptosis in naive, central and effector memory subsets of CD4+ and CD8+ T cells in aged humans. Exp Gerontol. 2008;43:266–274. doi: 10.1016/j.exger.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 53.Nuckel H, Frey UH, Roth A, Duhrsen U, Siffert W. Alemtuzumab induces enhanced apoptosis in vitro in B-cells from patients with chronic lymphocytic leukemia by antibody-dependent cellular cytotoxicity. Eur J Pharmacol. 2005;514:217–224. doi: 10.1016/j.ejphar.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 54.Gupta S, Gollapudi S. Effector memory CD8+ T cells are resistant to apoptosis. Ann N Y Acad Sci. 2007;1109:145–150. doi: 10.1196/annals.1398.017. [DOI] [PubMed] [Google Scholar]