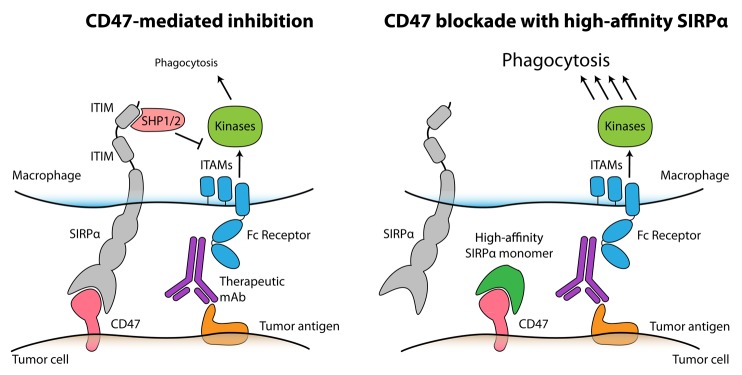
Figure 1. High-affinity SIRPα variants reduce the threshold for macrophage phagocytosis, thus enhancing the efficacy of anticancer monoclonal antibodies. Therapeutic monoclonal antibodies (mAbs) engage Fc receptors on the surface of macrophages, hence transducing positive signals via immunotyrosine-based activating motifs (ITAMs) and downstream mediators to stimulate phagocytosis. However, the phagocytic response of macrophages is limited by the expression of CD47 on tumor cells, as CD47 engages macrophage signal-regulatory protein α (SIRPα) to initiate an inhibitory signaling cascades mediated by immunotyrosine-based inhibitory motifs (ITIMs) and resulting in the activation of SHP-1 and SHP-2 phosphatases (left). The administration of high-affinity SIRPα monomers blocks CD47 on malignant cells and hence disables endogenous SIRPα signaling on macrophages. In combination with therapeutic antibodies, high-affinity SIRPα variants stimulate phagocytosis and hence mediate synergistic antitumor responses (right).
