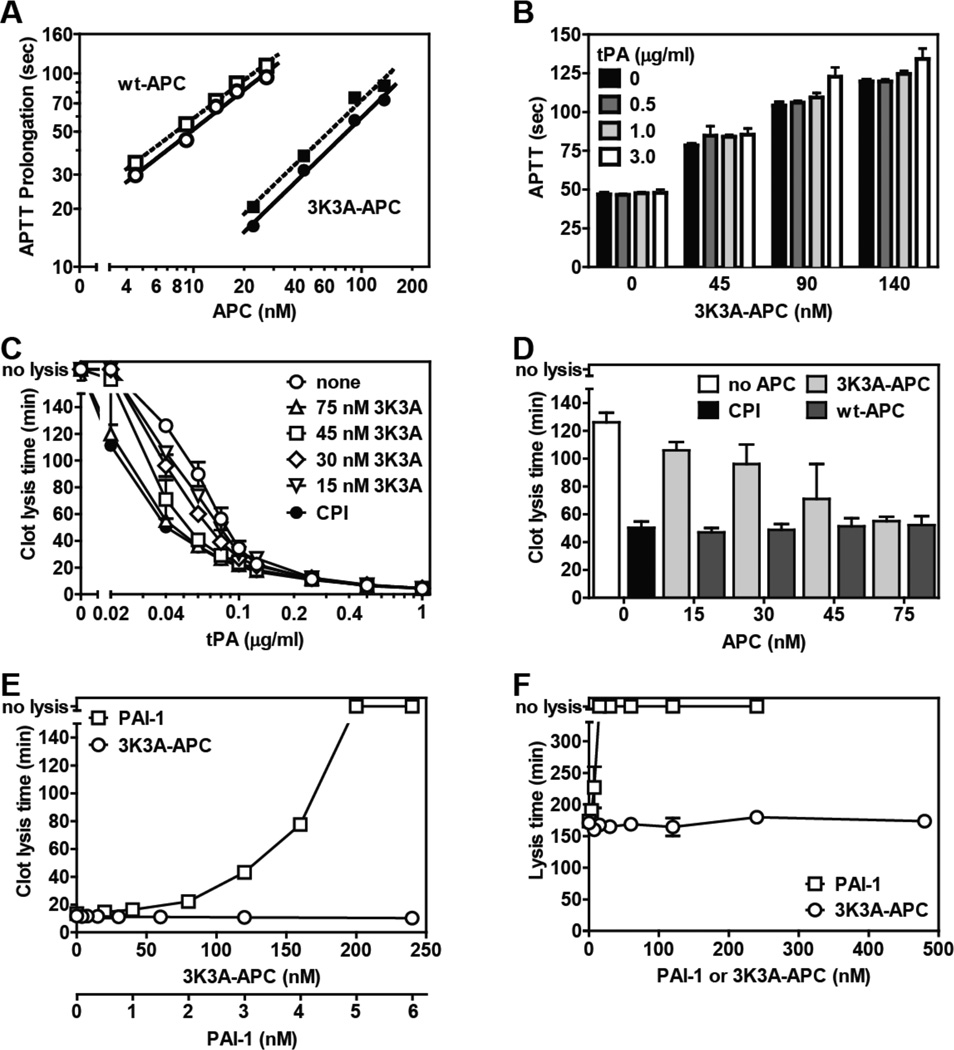
Figure. Reciprocal effects of tPA on 3K3A-APC’s anticoagulant activity and of 3K3A-APC on tPA’s fibrinolytic activity.
(A) Prolongation of the APTT (APTT-XL, Pacific Haemostasis) beyond the baseline reference clotting time of 47 sec was determined in protein C depleted plasma (Affinity Biologicals) for plasma-derived wt APC (Enzyme Research Laboratories) (open symbols) and for 3K3A-APC (ZZBiotech LLC) (solid symbols) when tPA (Innovative Research) was present at 0 (○,●) or 3.0 µg/ml (□,■). (B) The influence of tPA on the anticoagulant activity of 3K3A-APC was determined at the indicated tPA concentrations. (C) The effect of 3K3A-APC on tPA’s (Alteplase, Genentech) fibrinolytic activity was determined in clot lysis assays using thrombininduced clot formation and tPA-induced fibrinolysis in normal plasma (George King, Overland KS) as described [10]. CPI (10 µg/ml) was used to inhibit active TAFI activity where indicated (●). (D) Comparison of the profibrinolytic effect of recombinant wt-APC [10] and 3K3A-APC at 0.04 µg/ml tPA. (E) The ability of PAI-1 (stable mutant I91L, Molecular Innovations) and of 3K3A-APC to inhibit clot lysis induced by tPA (0.25 µg/ml) in the presence of CPI (10 µg/ml) which neutralizes TAFI activity. (F) The effect of 3K3A-APC or PAI-1 on tPA-mediated lysis of aged clots. Clots consisting of 50% normal plasma, 4 pM tissue factor (Innovin, Dade), phospholipids (10 µM, PC/PS/PE (60%/20%/20%)), 17 mM CaCl2 in HBS/BSA were aged for 2 hr after which 1.0 µg/ml tPA and 3K3A-APC were added to the surface of the clot to induce lysis. Data points represent mean values from n ≥ 3 experiments). “no lysis” on the y-axis indicates no clot lysis was observed.