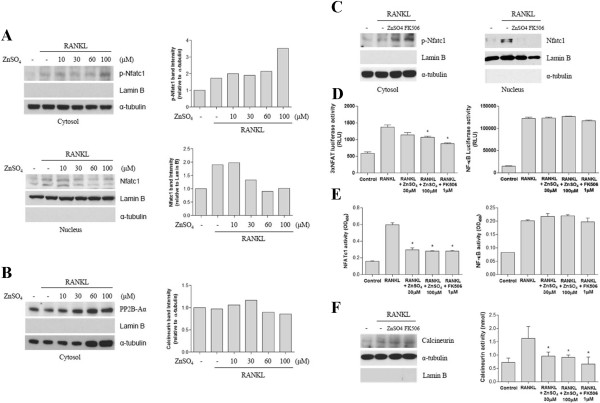
Figure 4.
Zinc Inhibits RANKL-induced Nfatc1 Activation by suppressing NFATc1 Translocation to the Nucleus in RAW264.7 cells. (A, B) RAW264.7 cells were incubated with RANKL (35 ng/ml) alone or RANKL (35 ng/ml) with various concentrations of ZnSO4. After 30 minutes, cytosolic and nuclear fractions were extracted from each group and evaluated by western blotting with the anti-phospho-Nfatc1 antibody (A, upper panel and C, left panel), anti-Nfatc1 antibody (A, lower panel, C, right panel), or anti-PP2B-Aα antibody (B), which is the catalytic subunit of calcineurin. Subcellular fraction purity and equal sample loading were evaluated by analyzing Lamin B and α-tubulin. Protein levels were quantified using densitometry. (C) RAW264.7 cells were incubated for 30 minutes with RANKL (35 ng/ml), RANKL (35 ng/ml) with ZnSO4 (100 μM), or RANKL (35 ng/ml) with FK506 (1 μM). Cytosolic phospho-Nfatc1 and nuclear Nfatc1 were analyzed using western blot. (D, E) RAW264.7 cells were stimulated with RANKL (R) or RANKL (R) plus ZnSO4 (30 or 100 μM) for 30 minutes. Nuclear fractions were prepared, and the transcriptional and DNA binding activity of Nfatc1 and NF-κB were measured using luciferase reporter assay and ELISA, respectively. RLU, Relative Light Units (F) RAW264.7 cells were cultured as shown in panel C. Cytosolic PP2B-Aα was examined by western blot and calcineurin activity was compared with the treated groups. Data are presented as the mean ± S.D. of three independent experiments; * p < 0.05 compared to RANKL (R).