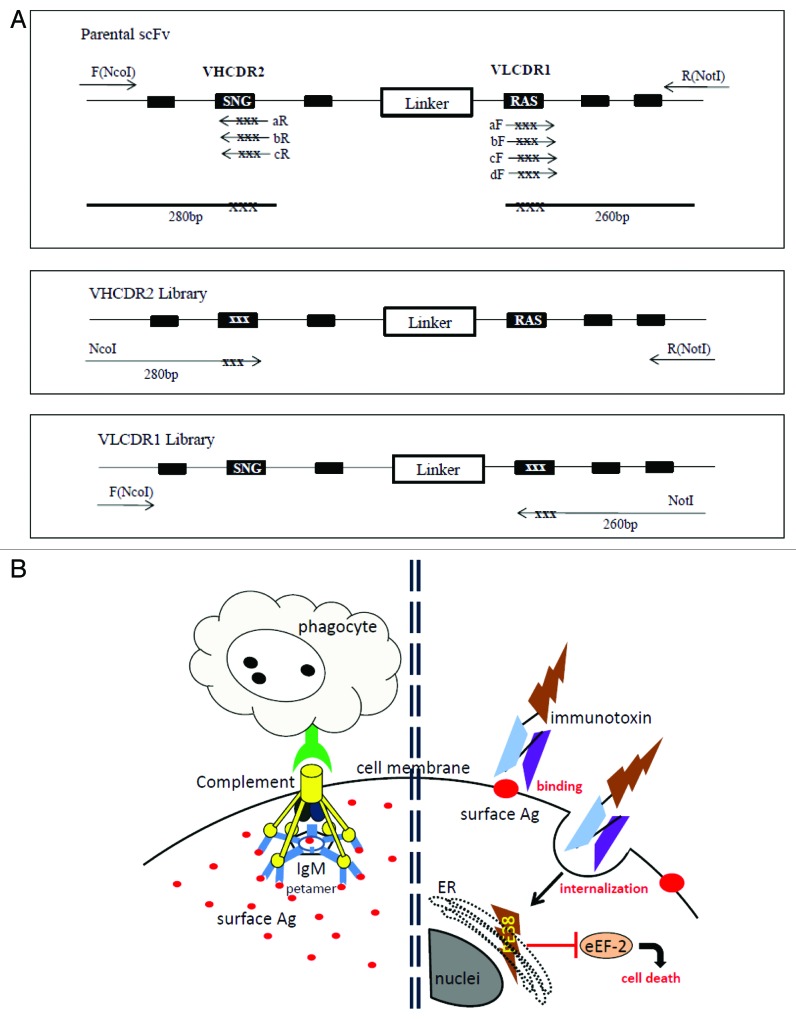
Figure 1. (A) PCR construction of mutant libraries. A primer fragment was first generated by using upstream DmAb14-scFv-F (NcoI) primer and downstream VHCDR2-aR, -bR, or -cR primers containing degeneracies (NNS) in the targeted VHCDR2 region. Another primer fragment was generated by using downstream DmAb14-scFv-R (NotI) and upstream VLCDR1-aF, -bF, -cF, or -dF primers containing degeneracies (NNS) in the targeted VLCDR1 region. DmAb14-scFv parental plasmid served as the template for amplification. The VHCDR2 fragments (280 bp) generated (panel A) and DmAb14-scFv-R(NotI) were paired and used as upstream and downstream primers (respectively) to generate the whole length of the scFv with VH mutation pool. Upstream DmAb14-scFv-F(NcoI) and downstream VLCDR1 (260 bp) primers, shown in panel A, were paired to generate a 750-bp scFv with a VL mutations library. (B) Different attacking mechanisms of IgM antibody and scFv-PE38KEDL RIT toward cancer cells. Left side of Figure 1B shows that IgM antibodies first form a pentamer to bind several identical epitopes on the cancer cells, and then need complements and/or phagocytes to fulfill the killing of the cancer cells19; Figure 1B (right side) displays the functioning mechanism of scFv-PE38KEDL RITs, which initializes by binding the surface epitope. Once the scFv-PE38KDEL RITs are internalized, the PE38KEDL toxin can eventually travel to the endoplasmic reticulum (ER) to cause the inactivation of eukaryotic translation elongation factor 2 (eEF2) by ADP-ribosylation, which results in translation inhibition and consequently cancer cell death.33

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.