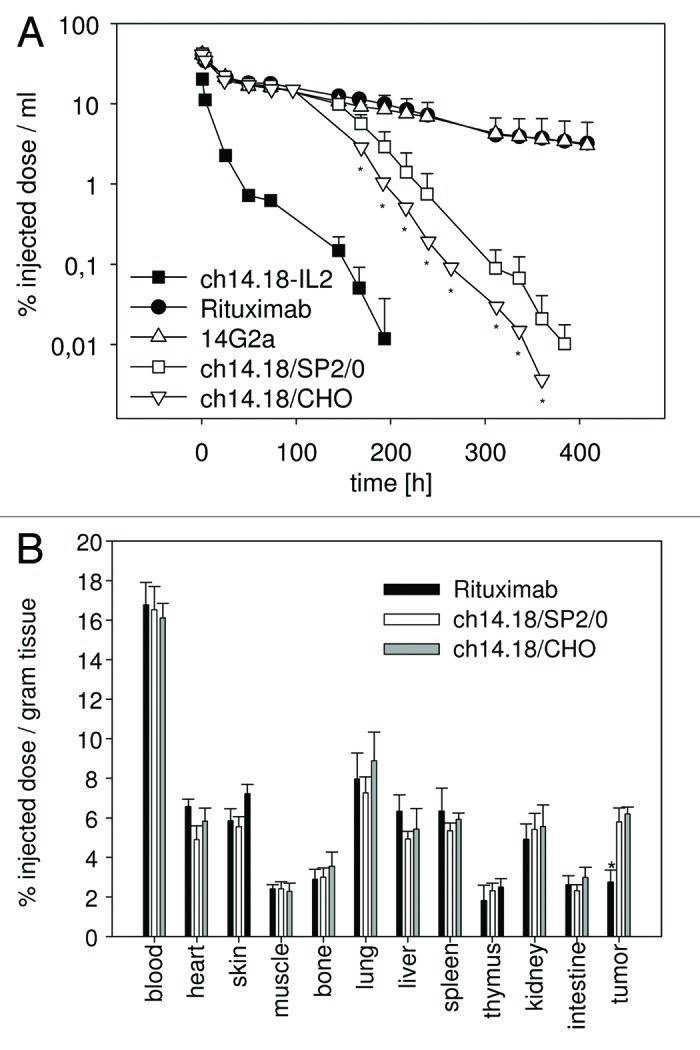
Figure 2. Clearance and biodistribution of ch14.18/CHO in mice. 125I-labeled ch14.18/CHO antibody (25 µg; 5 × 10E6 cpm) was injected into groups (n = 5) of Balb/c mice and neuroblastoma bearing A/J mice (average tumor weight 53 ± 12 mg) and clearance (A) as well as biodistribution (B) was analyzed and compared with indicated control antibodies. (A) The clearance of 125I-ch14.18/CHO from the blood of Balb/c mice was analyzed at indicated time points and compared with 125I-ch14.18/SP2/0. 125I-ch14.18-IL2, 125I-14G2a and 125I-rituximab are shown as controls. Blood levels of antibodies investigated are given as percent injected dose per milliliter of blood. Each value represents mean and standard deviation (n = 5). The t ½ α and β values were 25.0 h ± 1.9 h and 134.6 h ± 29.9 h for ch14.18 SP2/0 and 22.7 h ± 1.8 h and 136.2 h ± 12.2 h for ch14.18/CHO (p > 0.05). The rapid clearance of ch14.18-IL2 (human-mouse chimeric antibody interleukin-2 fusion protein) and the long half-life of 14G2 (murine IgG2a) and rituximab compared with ch14.18 (human-mouse chimeric antibody) are shown as controls. The difference between mice treated with ch14.18/CHO compared with controls was statistically significant (* p < 0.01). (B) Organ specific activity was detected 8 h after injection of 125I-ch14.18/CHO, 125I-ch14.18/SP2/0 and 125I-rituximab into neuroblastoma bearing A/J mice. Organ and tumor specific activity is given in percent of injected dose per gram of tissue. Each value represents the mean and standard deviation for five tumor bearing mice (* p < 0.01).
