Abstract
This study was done to clarify the clinical significance of vibration-induced nystagmus (VIN) and to calculate the sensitivity and the specificity of the vibration test. One hundred and twelve patients with unilateral peripheral vestibular disorders and thirty normal subjects were enrolled into this study. However, patients with spontaneous nystagmus were excluded. Vibration stimuli (approximately 100 Hz) were presented to the mastoids and the forehead. Patients and normal subjects also underwent head shaking nystagmus (HSN) test and caloric testing. Among the 112 patients, 91(81 %) showed VIN which were mainly horizontal. VIN was more frequently elicited on the mastoids than on the forehead. In the majority of patients (76 cases), the direction of VIN was toward the healthy side, whereas patients with Meniere’s disease (15 cases), showed nystagmus toward the affected side. None of 30 normal subjects showed VIN. Whereas HSN was found in 70 (63 %) patients and 9 (30 %) in normal subjects. Among 112 patients, 10 showed a canal paresis (CP) value of caloric testing less than 25 %, while 32 with a CP value between 25 and 40 %, 48 with a CP value between 40 and 70 %, and 22 with a CP value no less than 70 %. It is notable that with increasing CP value on caloric testing, VIN was more likely to be elicited. So VIN test is a simple, non-invasive and well-tolerated clinical test that indicates unilateral peripheral vestibular dysfunction. VIN test had greater sensitivity and specificity than HSN test in the diagnosis of unilateral peripheral vestibular disorders.
Keywords: Vibration-induced nystagmus (VIN), Caloric testing, Head shaking nystagmus (HSN), Unilateral vestibular dysfunction
Introduction
Up to now, the clinical examination methods of unilateral vestibular dysfunction have their own shortcomings [1, 2]. For example, caloric testing cannot be performed in patients with ear drum perforation [3]. Morever caloric testing can only evaluate low-frequency vestibular–ocular reflex (VOR) [3]. Although head shaking nystagmus (HSN) test can evaluate mid-frequency (about 2 Hz) VOR, it can’t be performed in patients with cervical spondylosis [4]. It has been reported that vibratory stimulus of the head and neck could induce nystagmus in patients with vestibular disorders while it couldn’t induce nystagmus in normal people. This vibration-induced nystagmus (VIN) has increasingly arose the interest of clinicians because that VIN test being an non-invasive, simple, fast and comfortable examination method specially suits for the bedside screening examination of the patients with vestibular disorders in clinic [5–8]. However there remains some controversy over the clinical significance and the usefulness of VIN [9]. Therefore, to clarify the clinical significance of VIN, we performed VIN test, HSN test and caloric testing in patients with unilateral vestibular disorders and normal subjects and observed their eye movements.
Materials and Methods
Patients Group
112 patients (54 men and 58 women; age range 8–75 years, average 43 years) with unilateral vestibular disorders came from vertigo clinic in department of otorhinolaryngology head and neck surgery, general hospital of Chinese people’s liberation army. They were diagnosed with definite Meniere’s disease (MD) [10] in 58 (left 32, right 26), vestibular neuritis [11] in 18 (left 10, right 8), recurrent vestibulopathy [12] in 10 (left 6, right 4), the vertigo accompanying sudden deaf [13] in 13 (left 6, right 7), vestibular involved inner ear malformation [14] in 5 (left dominant 3, right dominant 7), acoustic neuromas [15] before surgery in 2 (both right), labyrinth involved temporal bone fracture [16] in 2 (both right), delayed endolymphatic hydrops [17] in 2 (both right), idiopathic loss of inner ear function [18] in 2 (both right). The patients with spontaneous nystagmus, eardrum perforation and cervical spondylosis were excluded from the present study.
Normal Subjects Group
Thirty volunteers were used as control group, which include 24 men and 6 women with age range from 18 to 45 years and average age 28 years old. Those subjects have no complaints of vertigo, balance dysfunction, tinnitus and hearing loss.
Methods
All patients and normal subjects underwent VIN test, HSN test and caloric testing.
VIN Test
We used a hand-held vibration stimulator (mini vibrator NC70209, North Coast Medical, USA). The frequency of vibration was approximately 100 Hz, and the contact area was approximately 3 cm2. The vibrator was applied to the ipsilateral mastoid, contralateral mastoid and forehead for several seconds. During vibratory stimulation, eye movements in the sitting position were observed and recorded using videonystagmography (Micromedical Technologies Inc, USA).
HSN Test
The test was conducted as follows: 30° forward head flexion to position the lateral semicircular canals horizontally; rhythmical head rotation of 30–45° towards the two sides in the dark for 20–25 times at a frequency of 2 Hz. The test was considered positive when a nystagmus appeared: within the first 15–20 s after shaking or with a sequence of at least three clearly visible beats. We recorded the eye movements at least 1 min after ceasing of the first-phase nystagmus to reduce missing of biphasic nystagmus because of long latency.
Caloric Testing
Bithermal air caloric test was performed at temperatures of 50 and 24 °C using an apparatus from Micromedical Technologies (Chatham, IL, USA).Vestibular function can be divided into four grades according the canal paresis (CP) values in the caloric test results. The grades are as follows:
- Normal
CP value is less than 25 %;
- Mild lesion
CP value is no less than 25 % and less than 40 %;
- Moderate lesion
CP value is no less than 40 % and less than 70 %
- Profound lesion
CP is no less than 70 %
Statistical Analysis
Descriptive statistics and frequencies were determined for appropriate variables. Statistical analyses including χ2 test and Wilcoxon signed-rank test were performed using PASW Statistics version 17.0 (SPSS, Inc., Chicago, IL, USA). The level of significance was defined as p < 0.05.
Results
General Observation
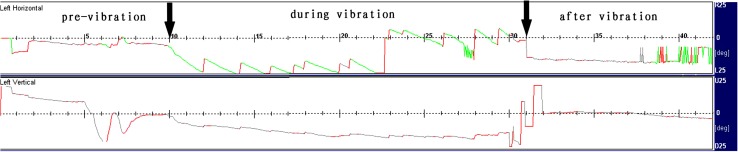
VIN was observed in 91 of 112 patients (81 %). When the vibratory stimulation was presented on the mastoid, the nystagmus appeared. It then disappeared when the vibratory stimulation was ceased (Fig. 1). The nystagmus was mainly horizontal in 91 patients and it was purely horizontal nystagmus in 84 patients while it has both horizontal and vertical nystagmus in 7 patients. No purely vertical nystagmus was observed. For the absence of three dimension eye movement recording device, the torsional components were not observed. Except for patients with MD, the VIN was directed toward the healthy side (Table 1). The VIN was directed toward the healthy side in 28 of the 58 patients with MD, whereas 15 patients showed VIN toward the affected side. At the same time VIN were not observed in all 30 healthy subjects. Hence the sensitivity and the specificity of VIN in the diagnosis of unilateral peripheral vestibular disorders were 81 % and 100 % respectively.
Fig. 1.
VIN of a 48-year-old woman with left acoustic neuroma. When the vibratory stimulation was presented on the left mastoid, right-beat nystagmus appeared. This nystagmus was disappeared when the vibratory stimulation was ceased. She had no caloric nystagmus by the left ear stimulation
Table 1.
VIN in patients with unilateral peripheral vestibular disorders
| Clinical diagnosis | VIN (+) | VIN (−) | Total | |
|---|---|---|---|---|
| Toward the healthy side | Toward the affected side | |||
| Meniere’s disease | 28 | 15 | 15 | 58 |
| Vestibular neuritis | 18 | 0 | 0 | 18 |
| Recurrent vestibulopathy | 8 | 0 | 2 | 10 |
| Vertigo accompanying sudden deaf | 10 | 0 | 3 | 13 |
| Vestibular involved inner ear malformation | 4 | 0 | 1 | 5 |
| Acoustic neuromas | 2 | 0 | 0 | 2 |
| Labyrinth involved temporal bone fracture | 2 | 0 | 0 | 2 |
| Delayed endolymphatic hydrops | 2 | 0 | 0 | 2 |
| Idiopathic loss of inner ear function | 2 | 0 | 0 | 2 |
| Total | 76 | 15 | 21 | 112 |
Sites Evoking Nystagmus
Nystagmus was often evoked by stimulation on the mastoids. Among the 91 patients who showed VIN, 89 patients showed VIN by stimulation on the mastoids. The VIN was evoked in only 21 patients by stimulation on the forehead. Eighty-one patients showed VIN by stimulation on the mastoid of the healthy side while 80 patients showed VIN by stimulation on the mastoid of the affected side. The majority of patients (72 of 91, 79 %) who showed VIN had VIN by stimulation on both mastoids (Table 2).
Table 2.
Sites evoking VIN
| Sites | Total | |
|---|---|---|
| All 3 sites | 19 | |
| Two of the 3 sites | Bilateral mastoids | 53 |
| Unilateral mastoid and forehead | 0 | |
| One of the 3 sites | Mastoid of the affected side | 8 |
| Mastoid of the unaffected side | 9 | |
| Forehead | 2 | |
| No nystagmus | 21 | |
| Total | 112 |
The Comparison of VIN with HSN
HSN was found in 70 of the 112 patients (63 %). Thirty patients showed VIN without HSN, whereas 9 patients showed HSN without VIN. Among the 61 patients who had HSN and VIN simultaneously, the directions of the VIN and HSN were identical in 52 patients (Table 3). Of 30 healthy subjects, 9 patients showed HSN and none showed VIN. Therefore the sensitivity and the specificity of HSN in the diagnosis of unilateral peripheral vestibular disorders were 63 % (70/112) and 70 % (21/30) respectively.
Table 3.
VIN versus head shaking nystagmus in patients with vestibular disorders
| Total | ||
|---|---|---|
| VIN (+), HSN (+) | Same direction | 52 |
| Different direction | 9 | |
| VIN (+), HSN (−) | 30 | |
| VIN (−), HSN (+) | 9 | |
| VIN (−), HSN (−) | 12 | |
| Total | 112 | |
VIN Versus Caloric Testing
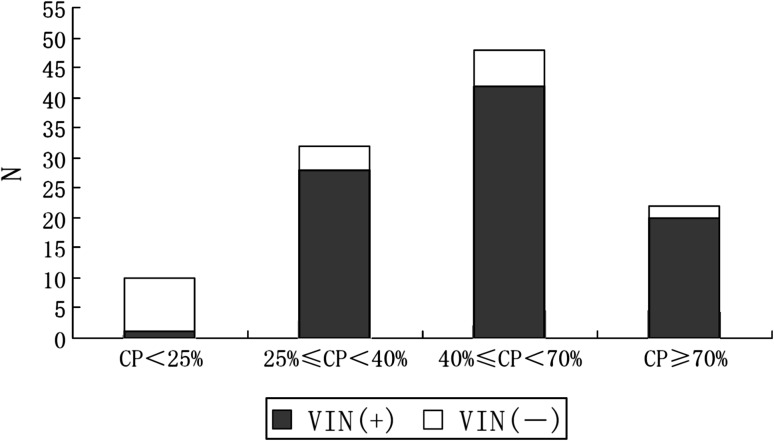
According to caloric testing, there were 10 normal, 32 mild lesions, 48 moderate lesions and 22 profound lesions in all 112 patients while there were all normal in 30 healthy subjects. Hence the sensitivity and the specificity of caloric testing in the diagnosis of unilateral peripheral vestibular disorders were 91 % (102/112) and 100 % (30/30). The positive rates of VIN in patients with CP of normal, mild lesion, moderate lesion and profound lesion were 10 % (1/10), 88 % (28/32), 88 % (42/48) and 91 % (20/22) (Fig. 2). Thus, the induction of VIN was significantly related to the degree of CP of the caloric testing; in other words, it was related to the degree of dysfunction in the lateral semicircular canal (p < 0.01, χ2 value is 2.848).
Fig. 2.
VIN versus caloric testing. Induction of VIN was significantly related to the degree of CP of the caloric testing (p < 0.01, χ2 test)
Discussion
VIN was often observed in patients with unilateral peripheral vestibular disorders, especially in patients with profound unilateral peripheral vestibular damages. Among patients with CP of more than 40 %, 89 % (62/70) of the patients showed VIN. On the other hand, only 10 % (1/10) of patients with CP of less than 25 % showed VIN. The present study demonstrated that the sensitivity and the specificity of VIN, HSN and caloric testing in the diagnosis of unilateral peripheral vestibular disorders were 81 % and 100 %, 63 % and 70 % as well as 91 % and 100 % respectively. Compared with HSN, VIN has higher sensitivity and specificity and nearly close to caloric testing. Therefore, from a practical viewpoint, VIN testing is a good, easy and non-invasive bedside clinical test for the detection of asymmetrical peripheral vestibular damages, although it is not perfect [6, 19].
The different induction rates of VIN and HSN in the patients with unilateral peripheral vestibular disorders and in healthy subjects were probably involved with the different mechanisms for the induction of VIN and HSN.
Abnormal results with the head shaking test depend on (1) asymmetry in peripheral vestibular input, which is best elicited with high-speed head shaking owing to Ewald’s second law; (2) storage of this asymmetry activity in central structures during head shaking; and (3) decay of the stored activity after the head stops moving [4, 20, 21]. In the acute phase of the unilateral peripheral vestibular disorders, central velocity storage may be impaired; thus HSN may be absent or even in the wrong direction [22].
Vibration can stimulate bilateral labyrinths and activate the vestibular receptors [23]. The signal from them via the vestibular nerve will be asymmetric in patients with unilateral vestibular dysfunction. The asymmetric signals in the vestibular nuclei will then lead to VIN. This hypothesis is somewhat similar to that of HSN [22]. We also found that the direction of HSN and VIN is identical in the most of patients. However, the mechanism for induction of VIN will not be totally identical to that of HSN. VIN might be evoked by a signal from the proprioceptors of the neck muscle, as vibratory stimulation to the neck muscles can stimulate the proprioceptive system. The proprioceptive system in the neck is linked to the vestibular system and ocular system to maintain equilibrium and control eye movements. It was reported that cervical dorsal root projects to the vestibular and oculomotor nuclei. Muscle vibration activates the primary endings of the muscle spindles. The response of the muscle spindles to vibration shows an abrupt onset and offset [24]. VIN showed an abrupt onset and offset, and the VIN did not persist after vibratory stimulation ceased. So VIN depends less on central velocity storage because the nystagmus can be easily appreciated during stimulation, unlike HSN, which is seen only after the head stops shaking; also, VIN does not usually outlast the period of vibration. Michel et al. [25] reported that 85 % of the 47 patients with unilateral vestibular lesions showed VIN, which is same to our study. They speculated that VIN would be of 2 origins: the labyrinth and the neck muscle. Ulmer et al. [26] also thought that vibration could stimulate vestibular receptors and neck proprioceptors and preferentially activate vestibular receptors. Although further study is required to clarify the mechanism of VIN induction [5–8], it has been well known that vibration mostly stimulate peripheral vestibular receptors while HSN is affected by central velocity storage mechanism. Hence the central velocity storage mechanism may be the main reason for the difference of the sensitivity of VIN and HSN in the diagnosis of the unilateral peripheral vestibular disorders. The present study found that all 30 normal subjects didn’t show VIN and 9 of them showed HSN. It could be explained by the above-mentioned mechanism.
Moreover the differences for the sensitivity and the specificity of VIN test, HSN test and caloric testing in the diagnosis of unilateral peripheral vestibular disorders may also be involved with the frequency characteristic of VOR in response to different stimulus used in these tests [18]. It is generally considered that caloric testing evaluates VOR with lower frequencies (about 0.003 Hz) and HSN test evaluates VOR with the medium frequencies (2–3 Hz) while VIN test evaluates VOR with higher frequencies (about 100 Hz) [19, 27–30]. It is speculated that the vestibular dysfunction with high frequencies revealed by VIN and HSN tests may be independent of those vestibular dysfunction with lower frequencies showed by caloric testing [27, 30]. Hence the results of VIN test, HSN test and caloric testing in the patients with the unilateral peripheral vestibular dysfunction may vary, depending on the frequency bands of VOR injured in different patients with peripheral vestibular disorders. Dumas et al. [19, 27–30] pointed out that the combination of VIN test, HSN test and caloric testing have played an important role in the multi-frequencies analysis of vestibular function especially for the patients with incomplete vestibular lesions. Therefore, the combined application of the HSN test, VIN test, and caloric testing is preferable for the all-around evaluation of vestibular function.
However HSN test and caloric testing also have their own shortcomings [4, 20–22]. As to caloric testing, the stimuli are unphysiological and the technique is still somewhat uncomfortable to the patient, and is also time-consuming (average 10 min); in addition, it can’t be performed for patients with eardrum perforation [1–3]. As far as HSN test is concerned, it can’t be performed in patients with neck injury [4, 20–22]. VIN test, which has greater sensitivity and specificity in the diagnosis of the unilateral peripheral vestibular hypofunction, can overcome those above-mentioned shortcomings. So being a simple, non-invasive and well-tolerated clinical test that indicates unilateral peripheral vestibular dysfunction, VIN test is playing a more and more important role in the screening of vestibular function [4–8, 19, 27–30].
Finally, it was still worthwhile to note that although VIN toward the healthy side was observed in most patients, VIN toward the affected side was still observed in some patients with MD. Ohki et al. [6] also found the same phenomena. VIN toward the affected side might be pathognomonic for MD. Thus, VIN toward the affected side should be dealt separately from VIN toward the healthy side.
Conclusions
The induction of VIN was significantly related to the unilateral vestibular dysfunction indicated by caloric testing. VIN test had greater sensitivity and specificity than HSN test in the diagnosis of unilateral peripheral vestibular disorders. Being a simple, non-invasive and well-tolerated clinical bedside examination test, VIN test is useful to reveal a vestibular asymmetry.
References
- 1.Lang EE, McConn Walsh R. Vestibular function testing. Ir J Med Sci. 2010;179:173–178. doi: 10.1007/s11845-010-0465-7. [DOI] [PubMed] [Google Scholar]
- 2.Wuyts FL, Furman J, Vanspauwen R, Van de Heyning P. Vestibular function testing. Curr Opin Neurol. 2007;20:19–24. doi: 10.1097/WCO.0b013e3280140808. [DOI] [PubMed] [Google Scholar]
- 3.Brandt T, Strupp M. General vestibular testing. Clin Neurophysiol. 2005;116:406–426. doi: 10.1016/j.clinph.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Kim MB, Huh SH, Ban JH. Diversity of head shaking nystagmus in peripheral vestibular disease. Otol Neurotol. 2012;33:634–639. doi: 10.1097/MAO.0b013e31824950c7. [DOI] [PubMed] [Google Scholar]
- 5.Hamann KF, Schuster EM. Vibration-induced nystagmus—a sign of unilateral vestibular deficit. ORL J Otorhinolaryngol Relat Spec. 1999;61:74–79. doi: 10.1159/000027645. [DOI] [PubMed] [Google Scholar]
- 6.Ohki M, Murofushi T, Nakahara H, Sugasawa K. Vibration-induced nystagmus in patients with vestibular disorders. Otolaryngol Head Neck Surg. 2003;129:255–258. doi: 10.1016/S0194-5998(03)00529-1. [DOI] [PubMed] [Google Scholar]
- 7.Perez N. Vibration induced nystagmus in normal subjects and in patients with dizziness. A videonystagmography study. Rev Laryngol Otol Rhinol (Bord) 2003;124:85–90. [PubMed] [Google Scholar]
- 8.Park HJ, Shin JE, Lim YC, Shin HA. Clinical significance of vibration-induced nystagmus. Audiol Neurootol. 2008;13:182–186. doi: 10.1159/000113508. [DOI] [PubMed] [Google Scholar]
- 9.Oas JG, Cherian N. Vibration-induced nystagmus is not a reliable sign of a unilateral vestibular loss. Neurology. 2001;56:A34–A35. [Google Scholar]
- 10.Thorp MA, Shehab ZP, Bance ML, Rutka JA. AAO-HNS Committee on Hearing and Equilibrium. The AAO-HNS Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Menière’s disease: have they been applied in the published literature of the last decade? Clin Otolaryngol Allied Sci. 2003;28:173–176. doi: 10.1046/j.1365-2273.2003.00687.x. [DOI] [PubMed] [Google Scholar]
- 11.Park H, Hong SC, Shin J. Clinical significance of vibration induced nystagmus and head-shaking nystagmus through follow-up examinations in patients with vestibular neuritis. Otol Neurotol. 2008;29:375–379. doi: 10.1097/MAO.0b013e318169281f. [DOI] [PubMed] [Google Scholar]
- 12.Lee HK, Ahn SK, Jeon SY, Kim JP, Park JJ, Hur DG, Kim DW, Woo SH, Kang HS. Clinical characteristics and natural course of recurrent vestibulopathy: a long-term follow-up study. Laryngoscope. 2012;122:883–886. doi: 10.1002/lary.23188. [DOI] [PubMed] [Google Scholar]
- 13.Kitahara T, Takeda N, Nishiike S, Okumura S, Kubo T. Prognosis of inner ear periphery and central vestibular plasticity in sudden deafness with vertigo. Ann Otol Rhinol Laryngol. 2005;114:786–791. doi: 10.1177/000348940511401008. [DOI] [PubMed] [Google Scholar]
- 14.Kokai H, Oohashi M, Ishikawa K, Harada K, Hiratsuka H, Ogasawara M, Miyashita S, Terayama Y. Clinical review of inner ear malformation. Nippon Jibiinkoka Gakkai Kaiho. 2003;106:1038–1044. doi: 10.3950/jibiinkoka.106.1038. [DOI] [PubMed] [Google Scholar]
- 15.Leonetti JP. The diagnosis and management of acoustic neuromas: contemporary practice guidelines. Compr Ther. 1995;21:68–73. [PubMed] [Google Scholar]
- 16.Benitez JT, Bouchard KR, Lane-Szopo D. Pathology of deafness and disequilibrium in head injury: a human temporal bone study. Am J Otol. 1980;1:163–167. [PubMed] [Google Scholar]
- 17.Kamei T. Delayed endolymphatic hydrops as a clinical entity. Int Tinnitus J. 2004;10:137–143. [PubMed] [Google Scholar]
- 18.Agrup C, Luxon LM. Immune-mediated inner-ear disorders in neuro-otology. Curr Opin Neurol. 2006;19:26–32. doi: 10.1097/01.wco.0000194143.02171.46. [DOI] [PubMed] [Google Scholar]
- 19.Dumas G, Perrin P, Schmerber S. Nystagmus induced by high frequency vibrations of the skull in total unilateral peripheral vestibular lesions. Acta Otolaryngol. 2008;128:255–262. doi: 10.1080/00016480701477677. [DOI] [PubMed] [Google Scholar]
- 20.Asawavichiangianda S, Fujimoto M, Mai M, Desroches H, Rutka J. Significance of head-shaking nystagmus in the evaluation of the dizzy patient. Acta Otolaryngol. 1999;Suppl 540:27–33. doi: 10.1080/00016489950181152. [DOI] [PubMed] [Google Scholar]
- 21.Angeli SI, Velandia S, Snapp H. Head-shaking nystagmus predicts greater disability in unilateral peripheral vestibulopathy. Am J Otolaryngol. 2011;32:522–527. doi: 10.1016/j.amjoto.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Hain TC. Head-shaking nystagmus and new technology. Neurology. 2007;68:1333–1334. doi: 10.1212/01.wnl.0000261902.31303.cf. [DOI] [PubMed] [Google Scholar]
- 23.Young ED, Fernandez C, Goldberg JM. Responses of squirrel monkey vestibular neurons to audio-frequency sound and head vibration. Acta Otolaryngol (Stockh) 1977;84:352–360. doi: 10.3109/00016487709123977. [DOI] [PubMed] [Google Scholar]
- 24.Burke D, Hagbarth KE, Löfstedt L, Wallin BG (1976) The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiol 261:673–693 [DOI] [PMC free article] [PubMed]
- 25.Michel J, Dumas G, Lavieille JP, Charachon R. Diagnostic value of vibration-induced nystagmus obtained by combined vibratory stimulation applied to the neck muscles and skull of 300 vertiginous patients. Rev Laryngol Otol Rhinol (Bord) 2001;122:89–94. [PubMed] [Google Scholar]
- 26.Ulmer E, Chays A, Brémond G. Vibration-induced nystagmus: mechanism and clinical interest. Ann Otolaryngol Chir Cervicofac. 2004;121:95–103. doi: 10.1016/S0003-438X(04)95495-3. [DOI] [PubMed] [Google Scholar]
- 27.Dumas G, Perrin P, Schmerber S, Lavieille JP. Nystagmus and vibration test research of mechanisms, theoretical methods: on 52 cases of unilateral vestibular lesions. Rev Laryngol Otol Rhinol (Bord) 2003;124:75–83. [PubMed] [Google Scholar]
- 28.Dumas G, Lavieille JP, Schmerber S. Vibratory test and head shaking test and caloric test: a series of 87 patients. Ann Otolaryngol Chir Cervicofac. 2004;121:22–32. doi: 10.1016/S0003-438X(04)95487-4. [DOI] [PubMed] [Google Scholar]
- 29.Dumas G, De Waele C, Hamann KF, Cohen B, Negrevergne M, Ulmer E, Schmerber S. Skull vibration induced nystagmus test. Ann Otolaryngol Chir Cervicofac. 2007;124:173–183. doi: 10.1016/j.aorl.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Dumas G, Karkas A, Perrin P, Chahine K, Schmerber S. High-frequency skull vibration-induced nystagmus test in partial vestibular lesions. Otol Neurotol. 2011;32:1291–1301. doi: 10.1097/MAO.0b013e31822f0b6b. [DOI] [PubMed] [Google Scholar]