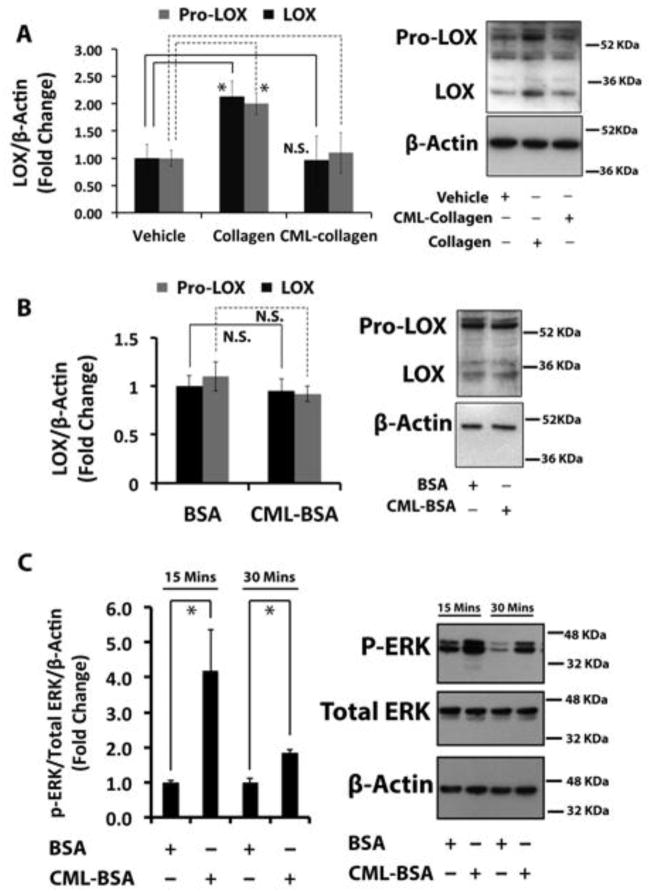
Figure 2. Collagen up-regulates lysyl oxidase in non-differentiated primary mouse osteoblasts, while glycated collagen fails to induce lysyl oxidase. The RAGE ligand CML-BSA does not down-regulate lysyl oxidase.
(A) Non-differentiated primary mouse osteoblasts were serum depleted and then treated with control collagen (0.2 mg/ml) or CML-collagen (glycated collagen; 0.2 mg/ml). Total protein was extracted and subjected to Western blotting for 50 kDa pro-lysyl oxidase and mature 32 kDa lysyl oxidase and β-actin (loading control). The bar graph shows regulation of both 50 kDa and 32 kDa lysyl oxidase protein levels in non-differentiated mouse primary osteoblasts in response to collagen or CML-collagen (n=3). Data are from one of three independent experiments with the same outcome. Data are mean ± SD (*, p<0.05, N.S., not significant; Student’s t-test). (B) Non-differentiated primary mouse osteoblasts were serum-depleted and then treated with BSA (0.2 mg/ml) or CML-BSA (0.2 mg/ml) for 24 hours and subjected to Western blotting for pro-lysyl oxidase and mature 32 kDa lysyl oxidase and β-actin (loading control). Data are representative of two independent experiments with the same outcome. Data are mean ± SD (N.S., not significant; Student’s t-test). (C) Primary calvarial mouse osteoblasts at 80% visual confluence were serum starved for 18 hours and treated with BSA or CML-BSA for either 15 or 30 minutes. Total protein from cell layers were collected and subjected to Western blotting to measure the phosphorylation levels of ERK, an indicator of AGE-RAGE axis activation. The graph shows the results of the densitometry analysis. Data are presented as means ± SD (n=3; *, p<0.05). Data are from one of two independent experiments with the same outcomes.