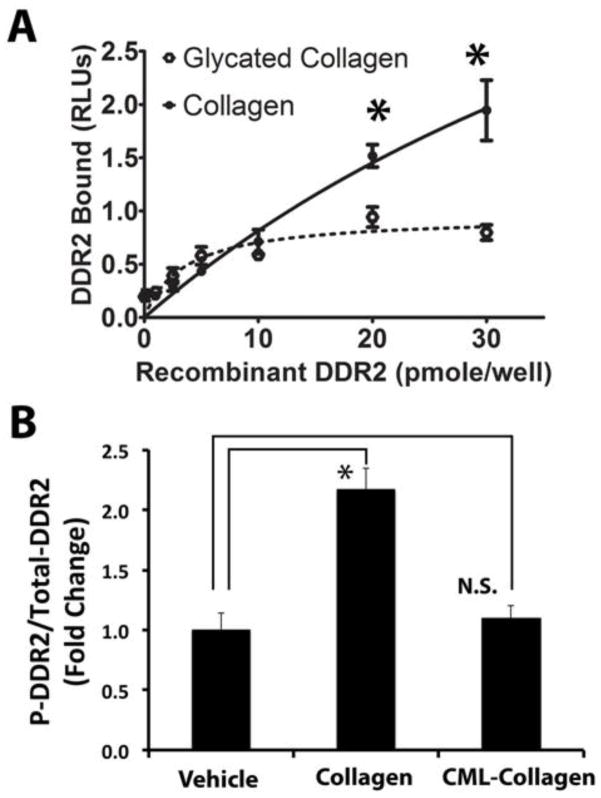
Figure 5. Glycation of collagen interferes with collagen-DDR2 binding and does not activate collagen-DDR2 signaling.
(A) Collagen or CML-collagen (10 μg/ml) was coated on wells of a 96-well plate. Increasing amounts of horseradish peroxidase-conjugated recombinant DDR2 was incubated for three hours at room temperature. The plate was washed and the relative levels of bound rDDR2 were measured utilizing a chromogenic substrate (Experimental Procedures). Data show binding curves for rDDR2 to collagen (solid line) and rDDR2 to CML-collagen (dotted line) and are means +/− SD (n = 6; p<0.05; Student’s t-test). Data are from one of two experiments with the same outcomes. (B) Non-differentiated primary mouse osteoblasts were serum-depleted and then treated with collagen (0.2 mg/ml). Total protein was extracted, and the phospho-DDR2 levels and total DDR 2 were measured using a phospho- and total-DDR2 ELISA kits. Collagen increased the ratio of phospho-DDR2 to DDR2, while CML-collagen did not induce DDR2 phosphorylation. Data are from one of two independent experiments with the same outcomes (n=3; *, p<0.05, N.S., not significant; Student’s t-test).