Abstract
Prior research indicates that disturbance of cholinergic neurotransmission reduces anxiety, leading to the hypothesis that people with heightened cholinergic function have a greater tendency toward anxiety-like and/or harm-avoidant behavior. We sought to determine if people with elevated levels of harm avoidance (HA), a dimension of temperament from the Temperament and Character Inventory (TCI), have high α4β2* nicotinic acetylcholine receptor (nAChR) availability. Healthy adults (n = 105; 47 non-smokers and 58 smokers) underwent bolus-plus-continuous infusion positron emission tomography (PET) scanning using the radiotracer 2-[18F]fluoro-3-(2(S)azetidinylmethoxy) pyridine (abbreviated as 2-FA). During the uptake period of 2-FA, participants completed the TCI. The central study analysis revealed a significant association between total HA and mean nAChR availability, with higher total HA scores being linked with greater nAChR availability. In examining HA subscales, both ‘Fear of Uncertainty’ and ‘Fatigability’ were significant, based on higher levels of these characteristics being associated with greater nAChR availabilities. This study adds to a growing body of knowledge concerning the biological basis of personality and may prove useful in understanding the pathophysiology of psychiatric disorders (such as anxiety disorders) that have similar characteristics to HA. Study findings may indicate that heightened cholinergic neurotransmission is associated with increased anxiety-like traits.
Keywords: Harm Avoidance, Temperament and Character Inventory, Tobacco, Nicotine Dependence, Positron Emission Tomography, Nicotinic Acetylcholine Receptor
1. Introduction
Biopsychosocial theories of personality propose that certain facets of temperament are linked to neurobiological (and genetic) markers (Cloninger, 1986, 1987). Personality dimensions influence both affective (Canli et al., 2001) and cognitive (Kumari et al., 2004) function as well as the risk of psychiatric disorders (Elovainio et al., 2004; Bora and Veznedaroglu, 2007; Smith et al., 2008), highlighting the importance of characterizing the biological basis of personality. Towards the end of the twentieth century, Robert Cloninger developed and presented the Temperament and Character Inventory, based on a psychobiological model of personality that describes the structure and diversity of personality characteristics using four dimensions of temperament (novelty seeking, harm avoidance [HA], reward dependence, and persistence) and three dimensions of character (cooperativeness, self-directedness, and self-transcendence) (Cloninger et al., 1993). The four dimensions of temperament are thought to be genetically and biologically determined and stable over time (Cloninger and Svrakic, 1997). Respectively, the four dimensions of temperament describe an individual’s propensity to pursue novelty, restrict behavior to avoid punishment, perform reward-related behaviors, and continue a behavior without reward. HA consists of the following subcharacteristics: anticipatory worry, fear of uncertainty, shyness, and rapid fatigability. Individuals rating high in HA tend to be pessimistic, cautious, and apprehensive (Cloninger et al., 1993; Pud et al., 2004), and HA is considered the most relevant of the four temperament dimensions to anxiety and affective disorders (Ampollini et al., 1999; Ball et al., 2002; Jiang et al., 2003).
Recently, increasing evidence implicates brain cholinergic neurotransmission in the modulation of harm-avoidant and anxiety-like behavior (Brioni et al., 1993; File et al., 2000; Newman et al., 2001). Neuronal nicotinic acetylcholine receptor (nAChR) antagonists such as mecamylamine (Newman et al., 2001; Newman et al., 2002; Lippiello et al., 2008; Zarrindast et al., 2008; Roni and Rahman, 2011), lobeline (Roni and Rahman, 2011), and methyllycaconitine (Tucci et al., 2003b) produce anxiolytic effects in animal models of anxiety. Furthermore, recent animal studies have demonstrated that partial and full agonists at nAChRs result in anxiolytic effects, suggesting that disturbance of brain cholinergic neurotransmission results in these effects (Brioni et al., 1993; Arneric et al., 1994; Brioni et al., 1994; Decker et al., 1994; Skoubis et al., 2006; Feuerbach et al., 2009; Turner et al., 2010). In addition, nicotine, acting as an agonist at nAChRs, can generate both anxiogenic and anxiolytic effects in animals (File et al., 1998; Ouagazzal et al., 1999; Picciotto et al., 2002; Graef et al., 2011) and humans (Picciotto et al., 2002; Tucci et al., 2003a; Graef et al., 2011; Kobiella et al., 2011) depending on the circumstance. Nicotine’s effects on anxiety behavior are influenced by dose, route of administration, acute or chronic dosing, time of testing, genetic background of animal subjects, and behavioral status (Picciotto et al., 2002). Taken together, this prior research indicates that disturbance of the cholinergic system may reduce anxiety, leading to the hypothesis that people with heightened cholinergic neurotransmission may have a greater tendency toward anxiety-like or harm-avoidant behavior.
Within the cholinergic system, the α4β2* nAChR subtype is one of the most abundant in the mammalian brain (Wu et al., 2006), and has been specifically linked with anxiety in animal models. In one such study, inactivation of β2*-containing nAChRs with a specific receptor antagonist reduced fear-like and anxiety-like behavior in rodents (Anderson and Brunzell, 2012). Similarly, β2*-containing nAChRs have been shown to be critical for the nicotine-induced enhancement of contextual fear conditioning (Wehner et al., 2004; Davis et al., 2007) and to mediate the anxiety-like and affective components of nicotine withdrawal (Jackson et al., 2008). In addition, α4-containing nAChRs have been shown to be necessary for the anxiolytic effects of nicotine (McGranahan et al., 2011). And, in a study of humans with major depressive disorder, both positive and negative associations were reported between β2*-containing nAChR availability and trait anxiety across a group of regions that differed from the ones studied here (Saricicek et al., 2012). Thus, these studies generally link the common α4β2* nAChR subtype with the mediation of anxiety-like or harm-avoidant behaviors.
Brain imaging studies of the dopaminergic, serotonergic, and opioid neurotransmitter systems have also examined links with HA. For dopaminergic neurotransmission, HA has been associated with high dopamine turnover (Kaasinen et al., 2001) and low dopamine receptor (D2/3) availability (Kim et al., 2011; Yasuno et al., 2001). For serotonergic neurotransmission, HA was found (in women) to be associated with increased 5-HT2A receptor binding potential (Bailer et al., 2004), and recent research has shown that serotonergic neurons in the dorsal raphe nucleus contain functional postsynaptic nAChRs, providing a mechanism by which the serotonergic system may influence nAChR density (Commons, 2008; Galindo-Charles et al., 2008). And for opioid neurotransmission, high HA score was shown to be associated with high μ-opioid receptor availability (implying low endogenous μ-opioid drive) (Tuominen et al., 2012). Taken together, these pioneering studies implicate high dopaminergic and low serotonergic and opioid neurotransmission in research participants with high HA. While dopaminergic, serotonergic, and opioid neurotransmission have begun to be characterized in relation to the personality trait HA, cholinergic neurotransmission has not yet (to our knowledge) been examined with brain imaging studies of humans.
Thus, we undertook a study to advance the characterization of the neuroreceptor profile of HA in a relatively large sample of healthy control participants. In the study presented here, we examined the relationship between HA and α4β2* nAChR availability in previously defined regions of interest (thalamus, cerebellum, brainstem, and prefrontal cortex) using high resolution positron emission tomography (PET) scanning.
2. Methods
2.1. Participants and Screening Methods
105 otherwise healthy adults (47 non-smokers and 58 smokers) completed the study and had usable data. Utilizing the same inclusion/exclusion criteria as in our previous reports (Brody et al., 2011; Brody et al., 2012), participants were recruited and screened, and the study sample here was a subset of the group used in a previous report by our group comparing smokers and non-smokers (Brody et al., 2012). For non-smokers, the central inclusion criterion was absence of cigarette smoking for at least the past year. For cigarette smokers, the central inclusion criteria were smoking 10–40 cigarettes per day and current nicotine dependence. Exclusion criteria for all study participants included: any history of substance abuse/dependence or mental illness, history of a medical condition or use of a medication that could affect central nervous system functioning during scanning, or pregnancy. Screening questions from the SCID-IV (First et al., 1995) were asked to participants, in order to rule out any history of substance abuse/dependence (other than nicotine dependence) and mental illness.
During an initial study visit, screening information was obtained to characterize smoking and other past history. Rating scales administered were: the Fagerström Test for Nicotine Dependence (FTND) (Fagerstrom, 1978; Heatherton et al., 1991), the Smoker’s Profile Form (including a detailed smoking history and demographic variables), the Hamilton Anxiety Rating Scale (HAM-A) (Hamilton, 1969), and the Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1967). To confirm smoking status, an exhaled carbon monoxide (CO) level was measured using a MicroSmokerlyzer (Bedfont Scientific Ltd, Kent, UK), with a CO ≥ 8 parts per million (ppm) considered consistent with active smoking status and a CO of ≤ 4 ppm being considered consistent with non-smoking status. A urine toxicology screen (Test Country I-Cup Urine Toxicology Kit), breathalyzer test (AlcoMatePro), and urine pregnancy test (for female participants of reproductive potential) (Test Country Cassette Urine Pregnancy Test) were obtained at the screening visit to verify the participant’s report of no current drug or alcohol dependence and no pregnancy. The local institutional review board (IRB) approved this study, and participants gave written informed consent.
2.2. Abstinence Period and Positron Emission Tomography (PET) Protocol
Approximately one week after the initial screening session, participants underwent PET scanning adhering to the same general procedure as in our previous reports (Brody et al., 2009; Brody et al., 2011; Brody et al., 2012). Smoker group participants began nicotine/smoking abstinence two nights before each PET session and were monitored as previously described (Brody et al., 2009; Brody et al., 2011). In this way, we attempted to minimize the effects of nicotine from smoking on PET radiotracer binding during scanning.
At 11AM on the day of scanning, study participants came to the VA Greater Los Angeles Healthcare System, and nicotine/smoking abstinence was confirmed by participant report and an exhaled CO ≤ 4 ppm. At 11:45AM, each participant had an intravenous line placed in a room next to the PET scanner. Bolus-plus-continuous-infusion of 2-FA was started at 12PM. The volume of 2-FA given as a bolus was equal to the volume infused over 500 minutes (Kboius = 500 min) (Kimes et al., 2008). This Kboius effectively reached an approximate steady state in past studies by our group and others (Kimes et al., 2008; Brody et al., 2009; Brody et al., 2011). After the bolus-plus-continuous-infusion was initiated, participants remained seated in the room next to the PET scanner for the next 4 h, allowing the radiotracer to come to a relatively steady state in the brain. At 4PM, PET scanning began and proceeded for 3 h, with a 10-min break following the first 90-min. Scans were obtained as series of 10-min frames.
PET scans were acquired with the Philips Gemini TruFlight (Koninklijke Philips Electronics N.V., Eindhoven, the Netherlands), a fully 3-dimensional PET-CT scanner, which was operated in non-TOF mode. Reconstruction was performed using Fourier rebinning and filtered back projection, and scatter and random corrections were applied. The mean spatial resolution (FWHM) for brain scanning is 5.0 mm (transverse) by 4.8 mm (axial). 2-FA was prepared using a published method (Dolle et al., 1998). Participants received a magnetic resonance imaging (MRI) scan of the brain within one week of PET scanning with the same specifications to those in our previous report (Brody et al., 2012).
During PET scanning, 5 mL blood samples were drawn to determine free, unmetabolized 2-FA and nicotine levels in plasma. For 2-FA levels, 4 samples were drawn as standards before 2-FA administration and 9 samples were drawn during PET scanning at predetermined intervals. 2-FA levels were measured using previously published methods (Shumway et al., 2007; Sorger et al., 2007). For nicotine levels, blood samples were obtained before and after PET scanning. After the samples were centrifuged, they were sent to Dr. Peyton Jacob’s laboratory at UCSF, where venous plasma nicotine concentrations were determined using an adapted version of a published GC-MS method (Jacob et al., 1991). The lower limit of quantification for this method was 0.2 ng/mL. In addition to the participants described in this paper, 19 smokers completed study procedures but were not included in the data analysis, as their plasma nicotine concentrations were unacceptably high (> 0.4 ng/mL) (determined after study participation).
2.3. Symptom Rating Scale Administration
In addition to baseline rating scales cited above, the Temperament and Character Inventory was administered once during the 2-FA uptake period (Cloninger et al., 1993), taking approximately 1 to 2 hours to complete.
2.4. PET Image Analysis
After motion and decay correction, participants’ PET scans were co-registered to their MRI images using PMOD version 2.9. Regions of interest (ROIs) were drawn on MRI using PMOD and transferred to the co-registered PET scans. ROIs were the thalamus, brainstem, cerebellum, and prefrontal cortex (PFC), which were chosen based on previous reports demonstrating a range of 2-FA receptor binding in these areas, while having at least moderate nAChR availability (Brody et al., 2006; Kimes et al., 2008; Mukhin et al., 2008). Mean VT/fP was used here for data analysis in order to limit Type I error, given that this study included a rating scale with 4 subscales and 4 ROIs, and because smokers have been consistently shown to have similar nAChR changes in almost all brain regions studied (Staley et al., 2006; Mamede et al., 2007; Mukhin et al., 2008; Wullner et al., 2008; Brody et al., 2012). The thalamus, cerebellum, and brainstem were drawn as whole structures, while representative slices of the PFC were drawn. ROI placement was visually examined for each PET frame to minimize effects of movement and co-registration errors. If a noticeable problem was detected, this procedure was repeated.
Total volume of distribution (expressed as VT/fP, based on established nomenclature (Innis et al., 2007)) was determined for each region and used as a measure of nAChR availability for the central study analyses. VT/fP values were calculated from the seventeen 10-minute PET frames as the ratio CT/(CP·fP), where CT is the total concentration of 2-FA in the ROIs, fP is the fraction of free (not protein bound) 2-FA in plasma, and (CP·fP) is the concentration of free 2-FA in plasma. The fraction of unmetabolized free (unbound) 2-FA was similar for the nonsmoker and smoker groups.
2.5. Statistical Analysis
For descriptive demographic variables, means (± standard deviations) of these data were determined. For the central study analysis, a univariate analysis of covariance (ANCOVA) was performed, with mean VT/fP values averaged across the 4 ROIs as the dependent variable, smoking status (smoker versus nonsmoker) as a fixed factor (because smoking is known to up-regulate nAChR levels across almost all brain regions studied), and total harm avoidance score as a covariate of interest. To clarify the significant finding from the preceding analysis, ANCOVAs were performed with the same variables using subscales of the overall harm avoidance scale as covariates of interest instead of total HA scores. For completeness, post hoc linear regression was performed within the smoker and non-smoker groups with mean VT/fP value as the dependent variable and total HA (and subscales) as independent variables, in order to clarify whether smoking status impacted the significant overall findings.
3. Results
The study sample was middle-aged (38.1 ± 12.8 years old), 60% male, and was representative of the west Los Angeles area in terms of race/ethnicity (54 Caucasians, 26 African-American, 10 Hispanic, and 15 Asian or Mixed). The sample had a mean 14.7 ± 2.1 years of education, drank 2.3 ± 3.3 alcoholic beverages per week, and drank 1.3 ± 1.5 coffee cup equivalents of caffeine per day. The sample had a mean total HA score of 8.4 ± 5.3 and minimal anxiety and depressive symptoms (HAM-A and HAM-D scores of 2.1 ± 2.3 and 1.8 ± 2.1, respectively). Eleven participants reported occasional marijuana use (≤ 2 uses per week). The smokers and non-smokers did not differ significantly in demographic or rating scale variables (Table 1). Consistent with prior research (Etter et al., 2003; Etter, 2010), smokers had slightly, but non-significantly (unpaired t test), higher total HA scores than non-smokers (total and subscale scores of 8.7 ± 5.1, 2.3 ± 1.8, 2.4 ± 1.6,1.8 ± 1.9, and 2.1 ± 2.0 for smokers and 8.1 ± 5.5, 2.2 ± 1.8, 2.6 ± 1.7,1.9 ± 2.1, and 1.4 ± 1.6 for non-smokers).
Table 1.
Demographic and Rating Scale Variables for the Overall Study Sample and Subgroups based on Smoking Status
| Variable | Study Sample (n = 105) | Smoker Subgroup (n = 58) | Non-Smoker Subgroup (n = 47) |
|---|---|---|---|
| Age | 38.1 (± 12.8) | 38.7 (± 13.4) | 37.4 (± 12.1) |
| Gender (% Female) | 40 | 36 | 45 |
| Ethnicity (% Caucasian) | 51 | 48 | 55 |
| Education (years) | 14.7 (± 2.1) | 14.4 (± 2.1) | 15.1 (± 2.1) |
| Cigarettes per day | N/A | 18.7 (± 4.2) | N/A |
| Caffeine (coffee cup equivalents) per day | 1.3 (± 1.5) | 1.6 (± 1.6) | 1.0 (± 1.3) |
| Alcoholic Beverages per week | 2.3 (± 3.3) | 2.8 (± 4.1) | 1.7 (± 2.0) |
| HAM-A Rating Scale Score | 2.1 (± 2.3) | 2.2 (± 2.4) | 2.0 (± 2.2) |
| HAM-D Rating Scale Score | 1.8 (± 2.1) | 2.0 (± 2.2) | 1.6 (± 2.0) |
The smoker and non-smoker subgroups did not differ significantly in any of the variables listed (unpaired t or chi-square tests nonsignificant) other than cigarettes per day. HAM-A = Hamilton Anxiety Rating Scale. HAM-D = Hamilton Depression Rating Scale.
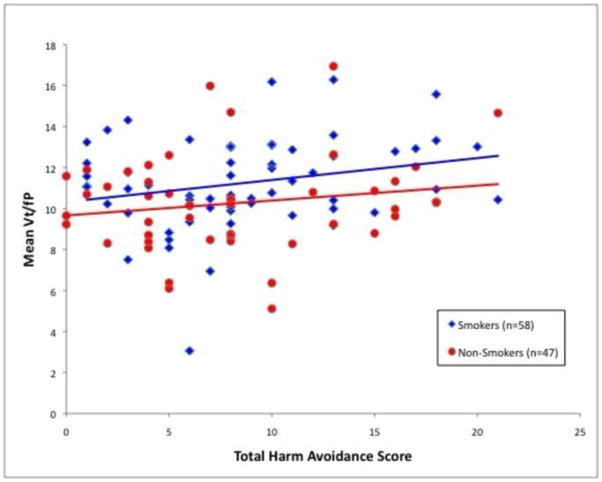
The central study analysis revealed a significant association between mean VT/fP values and total HA score (ANCOVA; df = 1,101; F = 4.7; P = 0.03), with higher VT/fP values being associated with greater total HA scores (Figures 1 and 2). This analysis also revealed the expected main effect of smoking status (ANCOVA; df = 1,101; F = 4.6; P = 0.04), with smokers having higher mean VT/fP values than non-smokers, presumably due to α4β2* nAChR up-regulation (Benwell et al., 1988; Breese et al., 1997). For this ANCOVA, the effect size (eta squared) was 0.088 of which 0.044 was from HA and 0.043 from smoking status.
Figure 1.
Significant association between mean Vt/fP (a marker for α4β2*availability) and total Harm Avoidance scores (ANCOVA; df = 1, 101; F = 4.7; P = 0.03). Similar results were found for the smoker and non-smoker subgroups.
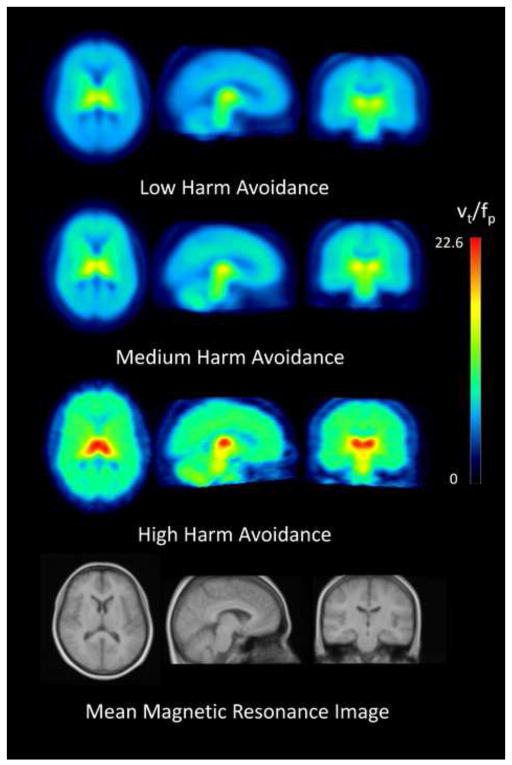
Figure 2.
Mean positron emission tomography (PET) images from study subgroups based on total Harm Avoidance (HA) scores, showing transaxial, saggital, and coronal views (columns 1 to 3). Top row shows the low HA group (range of scores 0 to 6; mean – 3.6), 2nd row shows medium HA (range of scores 7 to 9; mean – 7.9), and 3rd row show high HA (range of scores 10 to 25; mean – 14.0). Bottom row shows a mean magnetic resonance image of study participants.
As for the HA subscales, both ‘Fear of Uncertainty’ (ANCOVA; df = 1,101; F = 7.9; P = 0.006) and ‘Fatigability’ (ANCOVA; df = 1,101; F = 4.6; P = 0.03) were significant, with increasing VT/fP values being associated with higher levels of these measures. The other two subscales of HA (‘Anticipatory Worry’ and ‘Shyness’) did not have significant associations with nAChR availability (F’s = 1.2 and 0.2, respectively).
As for the subgroups of participants based on smoking status (smokers and non-smokers), these subgroups had similar directional relationships between VT/fP values and total HA (linear regression, P = 0.06 and P = 0.25, respectively), indicating that the overall result for total HA was similar for both smokers and non-smokers (Figure 1). Likewise, linear regression demonstrated that both subgroups also contributed to the significant findings for the HA subscales (‘Fear of Uncertainty’: P = 0.01 for smokers and P = 0.15 for non-smokers; ‘Fatigability’: P = 0.35 for smokers and P = 0.02 for non-smokers), indicating that the relationship between HA and nAChR availability was not dependent on smoking status.
4. Discussion
Study results demonstrate a positive association between mean nAChR availability in pre-defined ROIs (thalamus, cerebellum, brainstem, and PFC) and total scores for the personality trait Harm Avoidance. Two of the HA subscales (‘Fear of Uncertainty’ and ‘Fatigability’) were positively associated with mean nAChR availability, while one subscale (‘Anticipatory Worry’) had a non-significant positive association and the other subscale (‘Shyness’) had no association. The present study adds to a growing body of knowledge concerning the biological basis of personality. While we are not aware of brain imaging studies linking HA with nAChR availability, a prior behavioral genetics study found links between single nucleotide polymorphisms within the nAChR α4 subunit gene (CHRNA4) and HA (Roe et al., 2009), providing additional evidence for the mediation of HA by the cholinergic system. Furthermore, the findings here are consistent with prior research which demonstrates that the cholinergic system is linked with potentiation of a broad range of adaptive behaviors to environmental stimuli (Picciotto et al., 2012), which includes nAChR mediation of fear-like responses (Davis and Gould, 2007; Anderson and Brunzell, 2012), and also includes influences on attention, food intake, and affect.
In addition to cholinergic neurotransmission, previous studies have shown negative correlations between HA scores and dopamine D2/3 receptor availability in the pre-commissural dorsal caudate and post-commissural putamen (Kim etal., 2011), serotonin 5-HT(2) receptor availability in the frontal and left parietal cortex (Moresco et al., 2002), and serotonin transporter availability in the brainstem (Wu et al., 2010). In relation to the present study, these findings suggest that heightened cholinergic neurotransmission is associated with increased intrasynaptic dopamine and serotonin and diminished receptor availabilities. This theory is supported by previous studies demonstrating nicotine-induced dopamine release in the ventral striatum/nucleus accumbens using microdialysis (Di Chiara and Imperato, 1988; Damsma et al., 1989; Pontieri et al., 1996; Sziraki et al., 2001). Along these same lines, nicotine-induced dopamine release is inhibited when dopaminergic neurons are lesioned prior to nicotine administration (Corrigall et al., 1992). With respect to serotonergic neurotransmission, recent research has demonstrated that serotonergic neurons in the dorsal raphe nucleus possess functional postsynaptic nAChRs, providing a mechanism by which brain cholinergic neurotransmission may influence serotonin neurotransmission (Commons, 2008; Galindo-Charles etal., 2008).
Our results also support existing research implicating the thalamus, cerebellum, and PFC (regions specifically examined here) in the modulation of harm-avoidant behavior. Hakamata et al. reported a positive correlation with the level of glucose metabolism in the right medial dorsal thalamic nucleus, a region thought to modify emotion and mood (Hakamata et al., 2006). Additionally, O’Gorman et al. discovered a strong negative correlation between HA and perfusion in the cerebellar vermis (O’Gorman et al., 2006). Several studies have examined the role of the PFC in HA, and findings have included a negative correlation between HA and gray matter volume in the left PFC (Van Schuerbeek et al., 2011) and negative correlations between HA and glucose metabolism in the anterior ventromedial PFC (Hakamata et al., 2009) and left anterior PFC (Yamasue et al., 2008) in females. Furthermore, prior research has demonstrated significant activation of the dorsomedial PFC during internally driven uncertainty (Zaretsky et al., 2010) and right dorsolateral PFC during the anticipation of aversive stimuli (Nitschke et al., 2006), thereby linking PFC function with HA.
This study had several limitations. While the study size was substantial for a brain imaging study of this type, an even larger sample size would be needed to verify whether the smoker and non-smoker subgroups indeed had similar relationships between brain nAChR availability and HA, since these subgroup results were slightly different and it cannot be definitively concluded that the subgroups had the same relationship between nAChR availability and HA. Also, it should be noted that the Temperament and Character Inventory was administered during the 2-FA uptake period after two days of nicotine abstinence, and this timing may have had an unintended effect on the results from the smoker group given that their responses were collected in the setting of acute nicotine withdrawal. Furthermore, while we found a relationship between higher nAChR availability and higher HA, the causal relationship between nAChR availability and HA levels is unclear. And, while the TCI is a commonly-used, well-validated rating scale, the use of other personality rating scales, such as the NEO Personality Inventory-3 (NEO-PI-3), would have been helpful to confirm study results (McCrae et al., 2005).
Results of this study suggest that HA is associated with nAChR availability across brain regions and provide further evidence supporting a biological basis of personality. As personality dimensions influence the risk of psychiatric disorders, knowledge of biological and genetic determinants of personality may prove useful in understanding the pathophysiology of these conditions, and may (in the future) guide the development of new treatments for such disorders.
Acknowledgments
This study was supported by the Tobacco-Related Disease Research Program (A.L.B. [19XT-0135]), the National Institute on Drug Abuse (A.L.B. [R01 DA20872]), and the Department of Veterans Affairs (Merit Review Award [A.L.B.]). The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
The authors report no biomedical financial disclosures or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ampollini P, Marchesi C, Signifredi R, Ghinaglia E, Scardovi F, Codeluppi S, Maggini C. Temperament and personality features in patients with major depression, panic disorder and mixed conditions. Journal of Affective Disorders. 1999;52:203–207. doi: 10.1016/s0165-0327(98)00048-2. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Brunzell DH. Low dose nicotine and antagonism of beta2 subunit containing nicotinic acetylcholine receptors have similar effects on affective behavior in mice. PLoS One. 2012;7:e48665. doi: 10.1371/journal.pone.0048665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arneric SP, Sullivan JP, Briggs CA, Donnelly-Roberts D, Anderson DJ, Raszkiewicz JL, Hughes ML, Cadman ED, Adams P, Garvey DS, Wasicak JT, Williams M. (S)-3-methyl-5-(l-methyl-2-pyrrolidinyl) isoxazole (ABT 418): a novel cholinergic ligand with cognition-enhancing and anxiolytic activities: I. In vitro characterization. Journal of Pharmacology and Experimental Therapeutics. 1994;270:310–318. [PubMed] [Google Scholar]
- Bailer UF, Price JC, Meltzer CC, Mathis CA, Frank GK, Weissfeld L, McConaha CW, Henry SE, Brooks-Achenbach S, Barbarich NC, Kaye WH. Altered 5-HT(2A) receptor binding after recovery from bulimia-type anorexia nervosa: relationships to harm avoidance and drive for thinness. Neuropsychopharmacology. 2004;29:1143–1155. doi: 10.1038/sj.npp.1300430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball S, Smolin J, Shekhar A. A psychobiological approach to personality: examination within anxious outpatients. Journal of Psychiatric Research. 2002;36:97–103. doi: 10.1016/s0022-3956(01)00054-1. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJK, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3 H]nicotine binding sites in human brain. Journal of Neurochemistry. 1988;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Bora E, Veznedaroglu B. Temperament and character dimensions of the relatives of schizophrenia patients and controls: the relationship between schizotypal features and personality. European Psychiatry. 2007;22:27–31. doi: 10.1016/j.eurpsy.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S. Effect of smoking history on [3H]nicotine binding in human postmortem brain. Journal of Pharmacology and Experimental Therapeutics. 1997;282:7–13. [PubMed] [Google Scholar]
- Brioni JD, O’Neill AB, Kim DJ, Buckley MJ, Decker MW, Arneric SP. Anxiolytic- like effects of the novel cholinergic channel activator ABT-418. Journal of Pharmacology and Experimental Therapeutics. 1994;271:353–361. [PubMed] [Google Scholar]
- Brioni JD, O’Neill AB, Kim DJ, Decker MW. Nicotinic receptor agonists exhibit anxiolytic-like effects on the elevated plus-maze test. European Journal of Pharmacology. 1993;238:1–8. doi: 10.1016/0014-2999(93)90498-7. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Costello MR, Abrams AL, Scheibal D, Farahi J, London ED, Olmstead RE, Rose JE, Mukhin AG. Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. International Journal of Neuropsychopharmacology. 2009;12:305–316. doi: 10.1017/S146114570800922X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Khan A, Kozman D, Costello MR, Vellios EE, Archie MM, Bascom R, Mukhin AG. Effect of secondhand smoke on occupancy of nicotinic acetylcholine receptors in brain. Archives of General Psychiatry. 2011;68:953–960. doi: 10.1001/archgenpsychiatry.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, Koren AO, Mukhin AG. Cigarette smoking saturates brain alpha4beta2 nicotinic acetylcholine receptors. Archives of General Psychiatry. 2006;63:907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mukhin AG, La Charite J, Ta K, Farahi J, Sugar CA, Mamoun MS, Vellios EE, Archie MM, Kozman M, Phuong J, Arlorio F, Mandelkern MA. Up-Regulation of Nicotinic Acetylcholine Receptors in Menthol Cigarette Smokers. International Journal of Neuropsychopharmacology. 2012 doi: 10.1017/S1461145712001022. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JD. An fMRI study of personality influences on brain reactivity to emotional stimuli. Behavioral Neuroscience. 2001;115:33–42. doi: 10.1037/0735-7044.115.1.33. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatric Development. 1986;4:167–226. [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Archives of General Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM. Integrative psychobiological approach to psychiatric assessment and treatment. Psychiatry. 1997;60:120–141. doi: 10.1080/00332747.1997.11024793. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Archives of General Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Commons KG. Alpha4 containing nicotinic receptors are positioned to mediate postsynaptic effects on 5-HT neurons in the rat dorsal raphe nucleus. Neuroscience. 2008;153:851–859. doi: 10.1016/j.neuroscience.2008.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Damsma G, Day J, Fibiger HC. Lack of tolerance to nicotine-induced dopamine release in the nucleus accumbens. European Journal of Pharmacology. 1989;168:363–368. doi: 10.1016/0014-2999(89)90798-x. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. beta2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 2007;190:343–352. doi: 10.1007/s00213-006-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. Journal of Neuroscience. 2007;27:10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker MW, Brioni JD, Sullivan JP, Buckley MJ, Radek RJ, Raszkiewicz JL, Kang CH, Kim DJ, Giardina WJ, Wasicak JT, Garvey DS, Williams M, Arneric SP. (S)-3- methyl-5-(l-methyl-2-pyrrolidinyl)isoxazole (ABT 418): a novel cholinergic ligand with cognition-enhancing and anxiolytic activities: II. In vivo characterization. Journal of Pharmacology and Experimental Therapeutics. 1994;270:319–328. [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolle F, Valette H, Bottlaender M, Hinnen F, Vaufrey F, Guenther I, Crouzel C. Synthesis of 2-[F-18]fluoro-3-[2(S)-2-azetidinylmethoxy]pyridine, a highly potent radioligand for in vivo imaging central nicotinic acetylcholine receptors. Journal of Labelled Compounds & Radiopharmaceuticals. 1998;41:451–463. [Google Scholar]
- Elovainio M, Kivimaki M, Puttonen S, Heponiemi T, Pulkki L, Keltikangas-Jarvinen L. Temperament and depressive symptoms: a population-based longitudinal study on Cloninger’s psychobiological temperament model. Journal of Affective Disorders. 2004;83:227–232. doi: 10.1016/j.jad.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Etter JF. Smoking and Cloninger’s Temperament and Character Inventory. Nicotine Tobacco Research. 2010;12:919–926. doi: 10.1093/ntr/ntq116. [DOI] [PubMed] [Google Scholar]
- Etter JF, Pelissolo A, Pomerleau C, De Saint-Hilaire Z. Associations between smoking and heritable temperament traits. Nicotine Tobacco Research. 2003;5:401–409. doi: 10.1080/1462220031000094240. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring the degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Feuerbach D, Lingenhoehl K, Olpe HR, Vassout A, Gentsch C, Chaperon F, Nozulak J, Enz A, Bilbe G, McAllister K, Hoyer D. The selective nicotinic acetylcholine receptor alpha7 agonist JN403 is active in animal models of cognition, sensory gating, epilepsy and pain. Neuropharmacology. 2009;56:254–263. doi: 10.1016/j.neuropharm.2008.08.025. [DOI] [PubMed] [Google Scholar]
- File SE, Kenny PJ, Cheeta S. The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacology Biochemistry Behavior. 2000;66:65–72. doi: 10.1016/s0091-3057(00)00198-2. [DOI] [PubMed] [Google Scholar]
- File SE, Kenny PJ, Ouagazzal AM. Bimodal modulation by nicotine of anxiety in the social interaction test: role of the dorsal hippocampus. Behavioral Neuroscience. 1998;112:1423–1429. doi: 10.1037//0735-7044.112.6.1423. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders- Patient Edition (SCID-I/P, version 2.0) 1995. [Google Scholar]
- Galindo-Charles L, Hernandez-Lopez S, Galarraga E, Tapia D, Bargas J, Garduno J, Frias-Dominguez C, Drucker-Colin R, Mihailescu S. Serotoninergic dorsal raphe neurons possess functional postsynaptic nicotinic acetylcholine receptors. Synapse. 2008;62:601–615. doi: 10.1002/syn.20526. [DOI] [PubMed] [Google Scholar]
- Graef S, Schonknecht P, Sabri O, Hegerl U. Cholinergic receptor subtypes and their role in cognition, emotion, and vigilance control: an overview of preclinical and clinical findings. Psychopharmacology (Berl) 2011;215:205–229. doi: 10.1007/s00213-010-2153-8. [DOI] [PubMed] [Google Scholar]
- Hakamata Y, Iwase M, Iwata H, Kobayashi T, Tamaki T, Nishio M, Kawahara K, Matsuda H, Ozaki N, Honjo S, Inada T. Regional brain cerebral glucose metabolism and temperament: a positron emission tomography study. Neuroscience Letters. 2006;396:33–37. doi: 10.1016/j.neulet.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Hakamata Y, Iwase M, Iwata H, Kobayashi T, Tamaki T, Nishio M, Matsuda H, Ozaki N, Inada T. Gender difference in relationship between anxiety-related personality traits and cerebral brain glucose metabolism. Psychiatry Research. 2009;173:206–211. doi: 10.1016/j.pscychresns.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Diagnosis and rating of anxiety. British Journal of Psychiatry. 1969;3:76–79. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow and Metabolism. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. Journal of Pharmacology and Experimental Therapeutics. 2008;325:302–312. doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, 3rd, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3′,3′-d2 in humans. Biological Mass Spectrometry. 1991;20:247–252. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- Jiang N, Sato T, Hara T, Takedomi Y, Ozaki I, Yamada S. Correlations between trait anxiety, personality and fatigue: study based on the Temperament and Character Inventory. Journal of Psychosomatic Research. 2003;55:493–500. doi: 10.1016/s0022-3999(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Nurmi E, Bergman J, Eskola O, Solin O, Sonninen P, Rinne JO. Personality traits and brain dopaminergic function in Parkinson’s disease. Proceedings of the National Academy of Sciences USA. 2001;98:13272–13277. doi: 10.1073/pnas.231313198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Son YD, Kim HK, Lee SY, Cho SE, Kim YB, Cho ZH. Association of harm avoidance with dopamine D2/3 receptor availability in striatal subdivisions: a high resolution PET study. Biological Psychology. 2011;87:164–167. doi: 10.1016/j.biopsycho.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Kimes AS, Chefer SI, Matochik JA, Contoreggi CS, Vaupel DB, Stein EA, Mukhin AG. Quantification of nicotinic acetylcholine receptors in the human brain with PET: bolus plus infusion administration of 2-[18F]F-A85380. Neuroimage. 2008;39:717–727. doi: 10.1016/j.neuroimage.2007.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobiella A, Ulshofer DE, Vollmert C, Vollstadt-Klein S, Buhler M, Esslinger C, Smolka MN. Nicotine increases neural response to unpleasant stimuli and anxiety in non-smokers. Addiction Biology. 2011;16:285–295. doi: 10.1111/j.1369-1600.2010.00237.x. [DOI] [PubMed] [Google Scholar]
- Kumari V, ffytche DH, Williams SC, Gray JA. Personality predicts brain responses to cognitive demands. Journal of Neuroscience. 2004;24:10636–10641. doi: 10.1523/JNEUROSCI.3206-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippiello PM, Beaver JS, Gatto GJ, James JW, Jordan KG, Traina VM, Xie J, Bencherif M. TC-5214 (S-(+)-mecamylamine): a neuronal nicotinic receptor modulator with antidepressant activity. CNS Neuroscience & Therapeutics. 2008;14:266–277. doi: 10.1111/j.1755-5949.2008.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamede M, Ishizu K, Ueda M, Mukai T, Iida Y, Kawashima H, Fukuyama H, Togashi K, Saji H. Temporal change in human nicotinic acetylcholine receptor after smoking cessation: 5IA SPECT study. Journal of Nuclear Medicine. 2007;48:1829–1835. doi: 10.2967/jnumed.107.043471. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT, Jr, Martin TA. The NEO-PI-3: a more readable revised NEO Personality Inventory. Journal of Personality Assessment. 2005;84:261–270. doi: 10.1207/s15327752jpa8403_05. [DOI] [PubMed] [Google Scholar]
- McGranahan TM, Patzlaff NE, Grady SR, Heinemann SF, Booker TK. alpha4beta2 nicotinic acetylcholine receptors on dopaminergic neurons mediate nicotine reward and anxiety relief. Journal of Neuroscience. 2011;31:10891–10902. doi: 10.1523/JNEUROSCI.0937-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moresco FM, Dieci M, Vita A, Messa C, Gobbo C, Galli L, Rizzo G, Panzacchi A, De Peri L, Invernizzi G, Fazio F. In vivo serotonin 5HT(2A) receptor binding and personality traits in healthy subjects: a positron emission tomography study. Neuroimage. 2002;17:1470–1478. doi: 10.1006/nimg.2002.1239. [DOI] [PubMed] [Google Scholar]
- Mukhin AG, Kimes AS, Chefer SI, Matochik JA, Contoreggi CS, Horti AG, Vaupel DB, Pavlova O, Stein EA. Greater nicotinic acetylcholine receptor density in smokers than in nonsmokers: a PET study with 2–18F-FA-85380. Journal of Nuclear Medicine. 2008;49:1628–1635. doi: 10.2967/jnumed.108.050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MB, Manresa JJ, Sanberg PR, Shytle RD. Anxiolytic effects of mecamylamine in two animal models of anxiety. Experimental Clinical Psychopharmacology. 2002;10:18–25. doi: 10.1037//1064-1297.10.1.18. [DOI] [PubMed] [Google Scholar]
- Newman MB, Nazian SJ, Sanberg PR, Diamond DM, Shytle RD. Corticosterone- attenuating and anxiolytic properties of mecamylamine in the rat. Progress Neuropsychopharmacology Biological Psychiatry. 2001;25:609–620. doi: 10.1016/s0278-5846(00)00178-0. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- O’Gorman RL, Kumari V, Williams SC, Zelaya FO, Connor SE, Alsop DC, Gray JA. Personality factors correlate with regional cerebral perfusion. Neuroimage. 2006;31:489–495. doi: 10.1016/j.neuroimage.2005.12.048. [DOI] [PubMed] [Google Scholar]
- Ouagazzal AM, Kenny PJ, File SE. Stimulation of nicotinic receptors in the lateral septal nucleus increases anxiety. European Journal of Neuroscience. 1999;11:3957–3962. doi: 10.1046/j.1460-9568.1999.00823.x. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–129. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Pud D, Eisenberg E, Sprecher E, Rogowski Z, Yarnitsky D. The tridimensional personality theory and pain: harm avoidance and reward dependence traits correlate with pain perception in healthy volunteers. European Journal of Pain. 2004;8:31–38. doi: 10.1016/S1090-3801(03)00065-X. [DOI] [PubMed] [Google Scholar]
- Roe BE, Tilley MR, Gu HH, Beversdorf DQ, Sadee W, Haab TC, Papp AC. Financial and psychological risk attitudes associated with two single nucleotide polymorphisms in the nicotine receptor (CHRNA4) gene. PLoS One. 2009;4:e6704. doi: 10.1371/journal.pone.0006704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roni MA, Rahman S. Neuronal nicotinic receptor antagonist reduces anxiety-like behavior in mice. Neuroscience Letters. 2011;504:237–241. doi: 10.1016/j.neulet.2011.09.035. [DOI] [PubMed] [Google Scholar]
- Saricicek A, Esterlis I, Maloney KH, Mineur YS, Ruf BM, Muralidharan A, Chen JI, Cosgrove KP, Kerestes R, Ghose S, Tamminga CA, Pittman B, Bois F, Tamagnan G, Seibyl J, Picciotto MR, Staley JK, Bhagwagar Z. Persistent beta2*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. American Journal of Psychiatry. 2012;169:851–859. doi: 10.1176/appi.ajp.2012.11101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway DA, Pavlova OA, Mukhin AG. A simplified method for the measurement of nonmetabolized 2-[18F]F-A-85380 in blood plasma using solid-phase extraction. Nuclear Medicine Biology. 2007;34:221–228. doi: 10.1016/j.nucmedbio.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Skoubis PD, Hradil V, Chin CL, Luo Y, Fox GB, McGaraughty S. Mapping brain activity following administration of a nicotinic acetylcholine receptor agonist, ABT-594, using functional magnetic resonance imaging in awake rats. Neuroscience. 2006;137:583–591. doi: 10.1016/j.neuroscience.2005.08.072. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Cloninger CR, Harms MP, Csernansky JG. Temperament and character as schizophrenia-related endophenotypes in non-psychotic siblings. Schizophrenia Research. 2008;104:198–205. doi: 10.1016/j.schres.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger D, Becker GA, Patt M, Schildan A, Grossmann U, Schliebs R, Seese A, Kendziorra K, Kluge M, Brust P, Mukhin AG, Sabri O. Measurement of the alpha4beta2* nicotinic acetylcholine receptor ligand 2-[(18)F]Fluoro-A-85380 and its metabolites in human blood during PET investigation: a methodological study. Nuclear Medicine Biology. 2007;34:331–342. doi: 10.1016/j.nucmedbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, Dubin JA, Estok K, Brenner E, Baldwin RM, Tamagnan GD, Seibyl JP, Jatlow P, Picciotto MR, London ED, O’Malley S, van Dyck CH. Human tobacco smokers in early abstinence have higher levels ofbeta2* nicotinic acetylcholine receptors than nonsmokers. Journal of Neuroscience. 2006;26:8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sziraki I, Lipovac MN, Hashim A, Sershen H, Allen D, Cooper T, Czobor P, Lajtha A. Differences in nicotine-induced dopamine release and nicotine pharmacokinetics between Lewis and Fischer 344 rats. Neurochemistry Research. 2001;26:609–617. doi: 10.1023/a:1010979018217. [DOI] [PubMed] [Google Scholar]
- Tucci S, Genn RF, Marco E, File SE. Do different mechanisms underlie two anxiogenic effects of systemic nicotine? Behavioral Pharmacology. 2003a;14:323–329. doi: 10.1097/01.fbp.0000081782.35927.c6. [DOI] [PubMed] [Google Scholar]
- Tucci SA, Genn RF, File SE. Methyllycaconitine (MLA) blocks the nicotine evoked anxiogenic effect and 5-HT release in the dorsal hippocampus: possible role of alpha7 receptors. Neuropharmacology. 2003b;44:367–373. doi: 10.1016/s0028-3908(02)00391-x. [DOI] [PubMed] [Google Scholar]
- Tuominen L, Salo J, Hirvonen J, Nagren K, Laine P, Melartin T, Isometsa E, Viikari J, Raitakari O, Keltikangas-Jarvinen L, Hietala J. Temperament trait Harm Avoidance associates with mu-opioid receptor availability in frontal cortex: a PET study using [(ll)C]carfentanil. Neuroimage. 2012;61:670–676. doi: 10.1016/j.neuroimage.2012.03.063. [DOI] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. Nicotinic partial agonists varenicline and sazetidine-A have differential effects on affective behavior. Journal of Pharmacology and Experimental Therapeutics. 2010;334:665–672. doi: 10.1124/jpet.110.166280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schuerbeek P, Baeken C, De Raedt R, De Mey J, Luypaert R. Individual differences in local gray and white matter volumes reflect differences in temperament and character: a voxel-based morphometry study in healthy young females. Brain Research. 2011;1371:32–42. doi: 10.1016/j.brainres.2010.11.073. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, Beaudet A, Heinemann SF, Balogh SA. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Wu IT, Lee IH, Yeh TL, Chen KC, Chen PS, Yao WJ, Yang YK. The association between the harm avoidance subscale of the Tridimensional Personality Questionnaire and serotonin transporter availability in the brainstem of male volunteers. Psychiatry Research. 2010;181:241–244. doi: 10.1016/j.pscychresns.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Wu J, Liu Q, Yu K, Hu J, Kuo YP, Segerberg M, St John PA, Lukas RJ. Roles of nicotinic acetylcholine receptor beta subunits in function of human alpha4-containing nicotinic receptors. Journal of Physiology. 2006;576:103–118. doi: 10.1113/jphysiol.2006.114645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullner U, Gundisch D, Herzog H, Minnerop M, Joe A, Warnecke M, Jessen F, Schutz C, Reinhardt M, Eschner W, Klockgether T, Schmaljohann J. Smoking upregulates alpha4beta2* nicotinic acetylcholine receptors in the human brain. Neuroscience Letters. 2008;430:34–37. doi: 10.1016/j.neulet.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Abe O, Suga M, Yamada H, Inoue H, Tochigi M, Rogers M, Aoki S, Kato N, Kasai K. Gender-common and -specific neuroanatomical basis of human anxiety-related personality traits. Cerebral Cortex. 2008;18:46–52. doi: 10.1093/cercor/bhm030. [DOI] [PubMed] [Google Scholar]
- Zaretsky M, Mendelsohn A, Mintz M, Hendler T. In the eye of the beholder: internally driven uncertainty of danger recruits the amygdala and dorsomedial prefrontal cortex. Journal of Cognitive Neuroscience. 2010;22:2263–2275. doi: 10.1162/jocn.2009.21402. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Solati J, Oryan S, Parivar K. Effect of intra-amygdala injection of nicotine and GABA receptor agents on anxiety-like behaviour in rats. Pharmacology. 2008;82:276–284. doi: 10.1159/000161129. [DOI] [PubMed] [Google Scholar]