Figure 1.
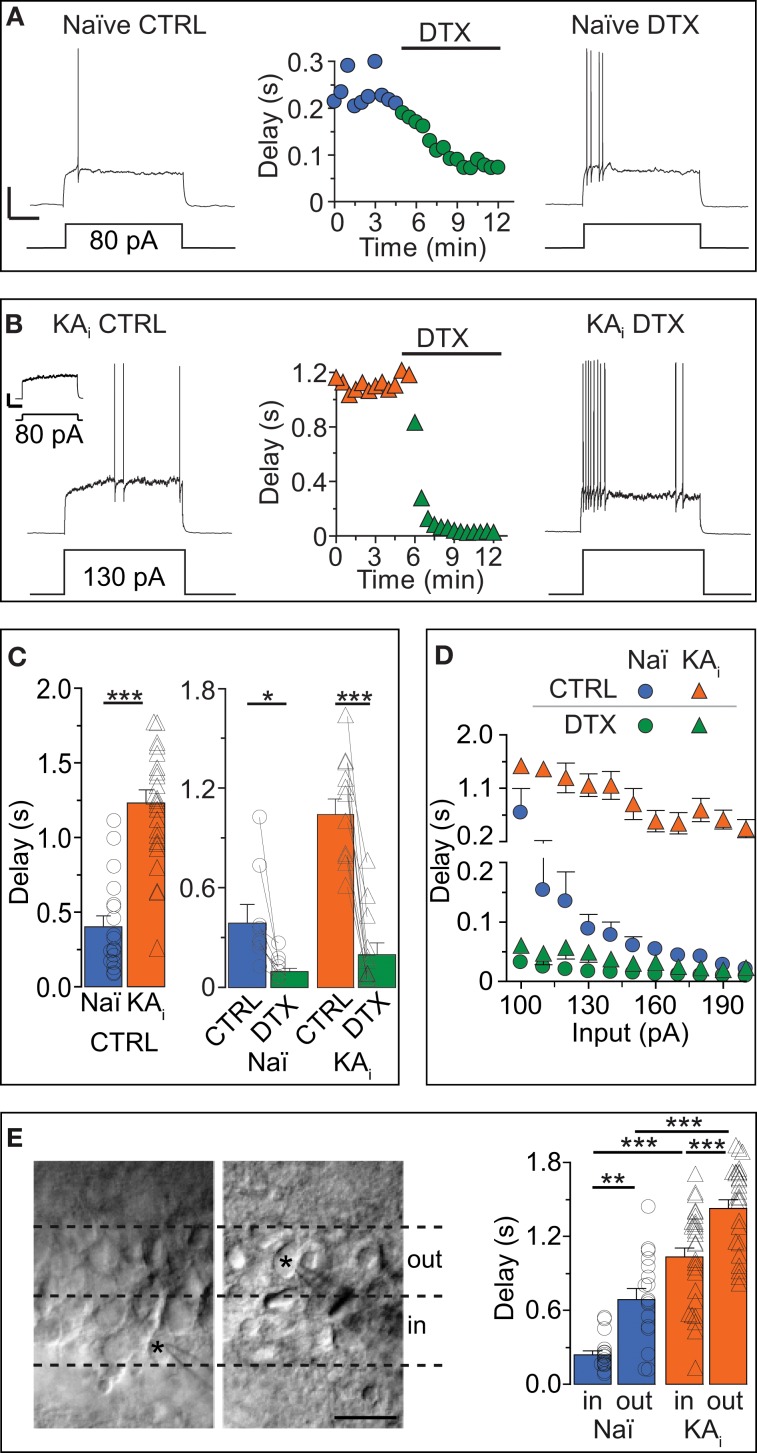
Dendrotoxin (DTX)-sensitive action potential response delays in DG cells of naïve mice and during hippocampal epilepsy. (A–C) Current-clamp recordings in DG cells of naïve [Naï, (A), circles] and KA-injected [KAi, (B), triangles] mice showing the transformation of a delayed action potential (AP) response under control condition (CTRL, left traces) into an almost immediate AP response by Kv1 channel blocker DTX (right traces). Because KA DG cells have a higher rheobase than Naï DG cells (Young et al., 2009), stronger current steps were injected into KA cells for pharmacological delay characterization [130 pA in (B)]. For direct comparison see inset in (B) [response of KA cell to current pulse as in (A), i.e., 80 pA]; Scale bars in (A,B) inset, 25 mV, 0.5 s. Compared to Naï DG cells, AP delays of KA DG cells were ~2.5 times prolonged (C). This was true with rheobase currents (C, right panel) and with current injections that were not statistically different (C, left panel). (D) Over a larger range of current injections, the response delays of KA DG cells were elevated (orange triangles) vs. Naï DG cells (blue circles) which displayed stronger response acceleration with increasing current injections. This difference was abolished with DTX (green triangles, KA; green circles, Naï, respectively). (E) Recorded DG cells localized via live fotos of patch pipettes (asterisks) were devided into inner DG cells (“in,” left panel, i.e., cells closer to the hilus outer) and outer DG cels (“out,” i.e., cells closer to the molecular layer). The grouping of recorded AP delays according to these areas (right panel) revealed on average longer delays in the outer DG cells from both naïve and KA-injected mice.