Figure 4.
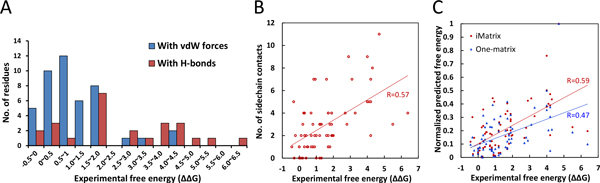
The evaluation of iMatrix on 70 mutations from the ASEdb. (A) The distribution of binding energy changes (ΔΔG) based on 70 mutated residues of antigen-antibody interfaces recorded in ASEdb. The mean is 2.54 and standard deviation is 1.84 of the binding free energy for 25 residues forming hydrogen bonds (red bars). Conversely, the mean is 1.08 and standard deviation is 1.03 of 45 residues forming vdW interactions (blue bars). (B) Distribution of free energies for the residues on sidechain interactions. The residues forming more side-chain contacts are often more influenced during the residue mutated into alanine. Pearson correlation coefficient is 0.57 between the ΔΔG and the number of side-chain contact. (C) The Pearson correlation coefficient are 0.59 and 0.47 between 70 experimental free energies (ΔΔG, recorded in ASEdb) and computational scores using iMatrix (red spot) and one-matrix (blue triangle), respectively.
