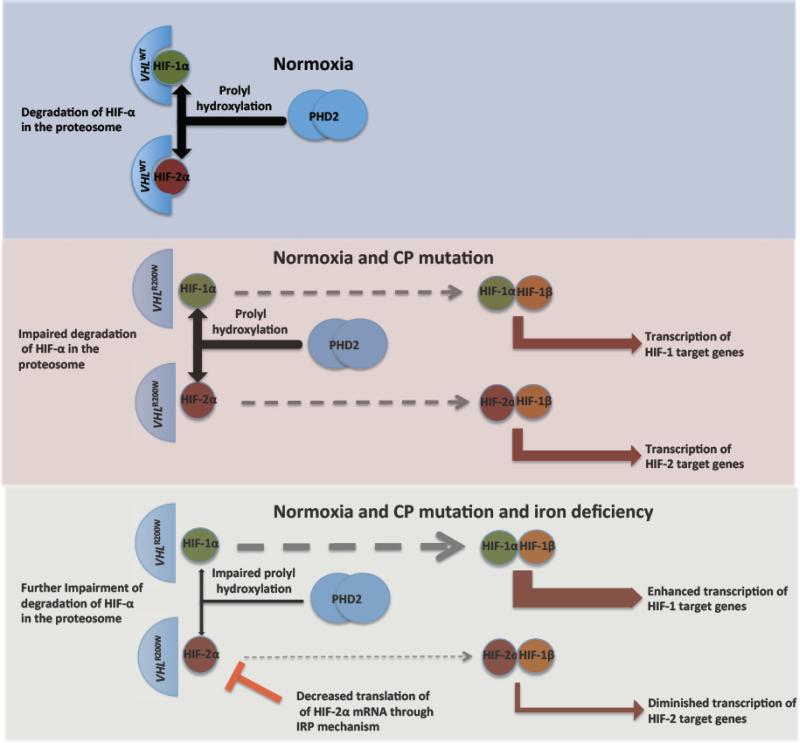
Figure 6.
Proposed mechanism for divergent expression of HIF-target genes according to iron status. Upper panel: With non-mutated VHL and normal iron status in normoxia, the alpha subunits of HIF are degraded in the proteasome after prolyl hydroxylation through the action of PHD2. Middle panel: The homozygous VHLR200W mutation causes impaired recognition of hydroxylated alpha-subunits of HIF, leading to increased levels of the alpha subunits of HIF and increased transcription of HIF target genes under normoxia. Lower panel: The addition of iron deficiency leads to a further increase in HIF-1α through impaired function of PHD2, but a decrease in HIF-2α because of reduced translation of HIF-2α through the interaction of iron responsive protein (IRP) with an iron response element in the 5’ untranslated region of HIF-2α mRNA.