Figure 2.
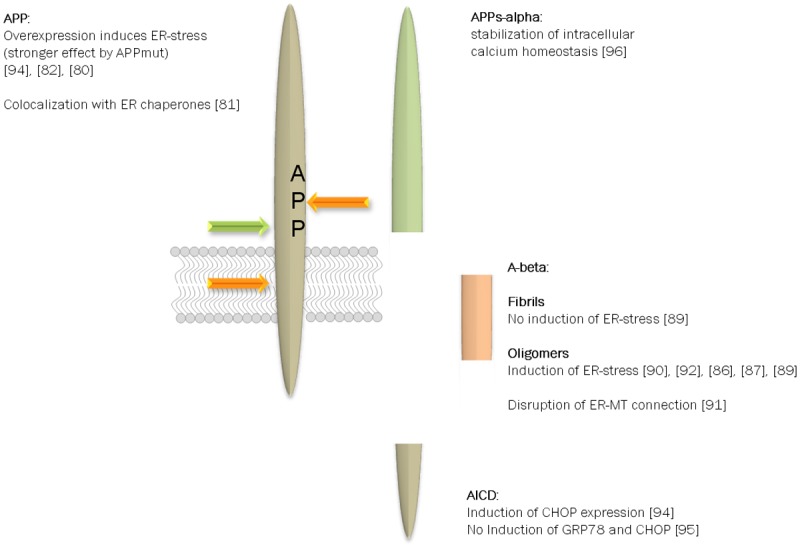
APP processing products and their potential role in UPR. APP is subject to a wide variety of posttranslational modifications such as phosphorylation and glycosylation (e.g. [62,155]). In addition, the type I transmembrane protein is cleaved by several proteases yielding protein fragments of different size and function [14]. In regard of ER-stress not all protein fragments have been analyzed but for the full length protein, A-beta peptides and the alpha-secretase derived soluble ectodomain correlation to subsequent signaling events have been reported. In this scheme only the most prominent facts are included but one has to consider that contradictory results have been published.
