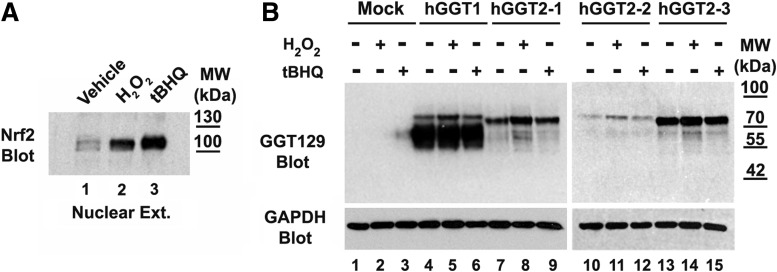
FIG. 5.
Oxidative stress does not induce cleavage of hGGT2 propeptides into heterodimers. (A) Nuclear lysates (10 μg total protein) from vehicle-treated HEK293T cells (lane 1), HEK293T cells cultured for 4 h in 250 μM H2O2 (lane 2) or 50 μM tBHQ (lane 3) were subjected to SDS-PAGE and immunoblotted with an antibody specific for Nrf2. Presence of Nrf2 in the nucleus of the H2O2 and tBHQ-treated cells confirms the induction of oxidative stress under these treatment conditions. (B) HEK293T cells were transiently transfected with empty vector (lanes 1–3), hGGT1 cDNA (lanes 4–6), hGGT2-1 cDNA (lanes 7–9), hGGT2-2 cDNA (lanes 10–12), or hGGT2-3 cDNA (lanes 13–15) expression constructs. Twenty-four hours after transfection, vehicle (ethanol), 250 μM H2O2, or 50 μM tBHQ was added to the cells. After 24 h, whole cell lysates were resolved on an 8% SDS-polyacrylamide gel. Resolved proteins were electroblotted onto nitrocellulose and then subjected to immunoblotting, using the GGT129 antibody, which recognizes a common epitope in hGGT1 and each of the hGGT2 alleles. The antibody identifies both the propeptide and large subunit of hGGT1. hGGT2-1,2,3 are expressed but present only as a propeptide under each of these culture conditions. The weak signal observed in lane 3 is carried over from lane 4 as confirmed by independent blots and activity assays. H2O2, hydrogen peroxide; tBHQ, tert-butylhydroquinone.