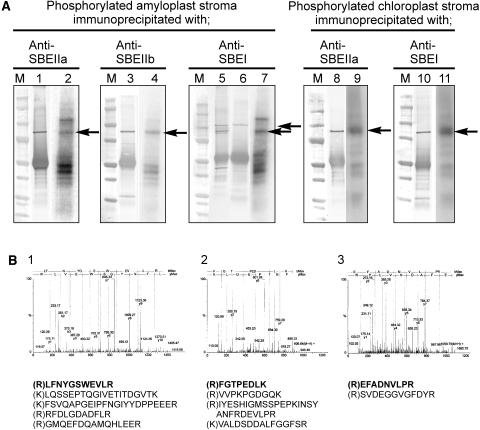
Figure 2.
Identification of the Phosphorylated 87- to 88-kD Polypeptides from Plastids as SBE Isoforms.
Intact amyloplasts (isolated from T. aestivum endosperm 12 to 25 DAP, 1.2 to 1.5 mg protein cm−3) and chloroplasts (isolated from 8- to 10-d-old T. aestivum leaves, 1.4 to 1.8 mg protein cm−3) were incubated with 100 μM γ-32P-ATP for 20 min at 25°C. Reactions were terminated, organelles lysed, and the stromal phosphoproteins partially purified by affinity chromatography as described in Methods.
(A) Stromal phosphoproteins were incubated with different, peptide-specific, anti-SBE antisera, and the 87- to 88-kD phosphoproteins were immunoprecipitated as described in Methods. Lanes 1, 3, 5, 8, and 10, immunoblots of the immunoprecipitated phosphoproteins probed with the respective anti-SBE antisera. Lane 6, an immunoblot of amyloplast stromal proteins immunoprecipitated with anti-SBEI antibodies and probed with anti-H. vulgare endosperm (plastidial) starch phosphorylase antbodies. Lanes 2, 4, 7, 9, and 11, autoradiographs of the respective immunoblots. M, molecular mass markers. Arrows indicate the positions of the phosphorylated 87- to 88-kD polypeptides that cross-reacted with the respective anti-SBE antibodies.
(B) Q-TOF-MS data of peptides from the 87- to 88-kD phosphoproteins immunoprecipitated with different anti-SBE antibodies and separated by 2D-PAGE. Panels 1 to 3, the MS survey acquisition data obtained for the 87- to 88-kD phosphoproteins from amyloplast stroma immunoprecipitated with the following: 1, anti-SBEIIa antibodies; 2, anti-SBEIIb antibodies; and 3, anti-SBEI antibodies. The data presented are for single representative analyses and in each case show the spectra obtained for one of the peptides. Below them are the corresponding sequences from each spectrum (in bold) and the sequences of other peptides acquired from the same sample.