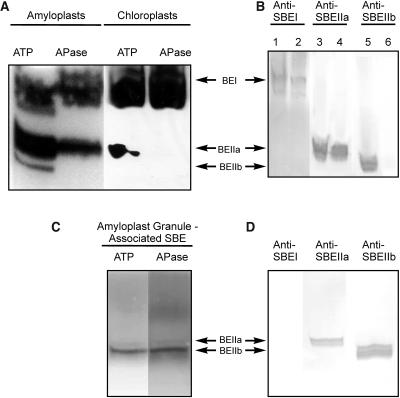
Figure 4.
Activity Gel Analysis of SBE Isoforms Showing Effects of Dephosphorylation on Enzyme Activity.
(A) Zymogram analysis of SBE activity from stromal extracts of amyloplasts (isolated from endosperm 12 to 25 DAP) and chloroplasts (from 8- to 10-d-old leaves) after pretreatment of the samples with 1 mM ATP or 10 units APase for 20 min at 25°C. Approximately 80 μg of amyloplast stromal protein per lane and 120 μg of chloroplast stromal protein per lane were separated on a 7-cm native polyacrylamide gel containing substrates for SBE. SBE activities were visualized by staining with I2/KI.
(B) Immunoblots from SBE zymogram gels of amyloplast and chloroplast stromal proteins (same protein loadings as in [A]) developed with antisera against SBEI (lanes 1 and 2), SBEIIa (lanes 3 and 4), and SBEIIb (lanes 5 and 6), showing the positions of each of the different SBE forms on the zymogram. The antisera used in the immunoblots in Figure 4B were used at the same dilutions as for the immunoprecipitation experiments. Lanes 1, 3, and 5 contain amyloplast proteins, and lanes 2, 4, and 6 contain chloroplast proteins.
(C) Zymogram analysis of granule-associated SBE activity in amyloplasts. Starch granule–associated proteins were extracted after incubation of plastids with ATP and subsequently either untreated or incubated with APase (see above). Samples were separated on native gels (∼80 μg of protein per lane) and SBE activity visualized as above.
(D) Immunoblots from SBE zymogram gels of granule-associated proteins from amyloplasts (ATP treated) developed with various anti-SBE antisera as shown.