Abstract
DNA glycosylase AlkD excises N7-methylguanine (7mG) by a unique but unknown mechanism, in which the damaged nucleotide is positioned away from the protein and the phosphate backbone distorted. Here, we show by methylphosphonate substitution that a phosphate proximal to the lesion has a significant effect on the rate enhancement of 7mG depurination by the enzyme. Thus, instead of a conventional mechanism whereby protein side chains participate in N-glycosidic bond cleavage, AlkD remodels the DNA into an active site composed exclusively of DNA functional groups that provide the necessary chemistry to catalyze depurination.
DNA glycosylases liberate aberrant nucleobases from the genome by catalyzing the hydrolysis of the N-glycosidic bond. Most glycosylases flip the target nucleobase into an active site that contains conserved side chains necessary for general acid-base catalysis (Figure 1A).1 Kinetic isotope effects and quantum mechanical calculations for several monofunctional DNA and RNA glycosylases are consistent with a dissociative (DN*AN) mechanism involving a cationic oxocarbenium intermediate that is converted to an abasic site by a water nucleophile.2–7 Catalysis by a variety of glycosylases is driven largely by a conserved carboxylate side chain, which can electrostatically stabilize the oxocarbenium intermediate and/or activate the water nucleophile 8–11. In the case of purine excision, a general acid protonates N7 to activate the nucleobase leaving group.4–7,12–15 In addition to protein functional groups, the DNA backbone has been shown to play a role in base excision by several enzymes.4,7,16–18 The best studied example is human uracil DNA glycosylase, in which DNA phosphates promote glycosidic bond cleavage by stabilizing the charge or conformation of the oxocarbenium intermediate.4,7,16,17,19–21
Figure 1.
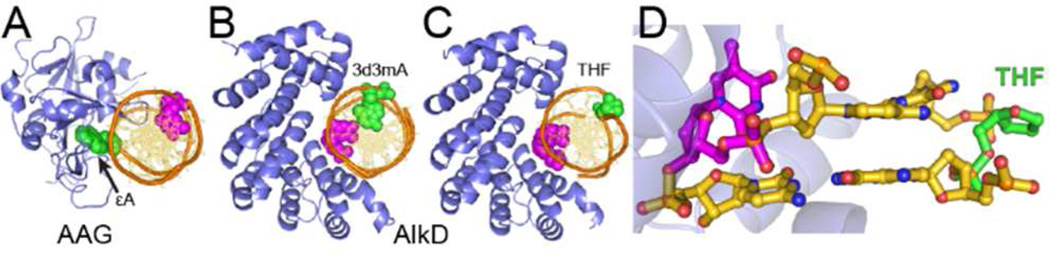
AlkD traps the lesion away from the protein. (A) Crystal structure of human alkyladenine DNA glycosylase (AAG) in complex with 1,N6-ethenoadenine (εA)-DNA. (B,C) Crystal structures of AlkD in complex with (B) 3-deaza-3-methyladenine (3d3mA)-DNA and (C) tetrahydrofuran (THF)-DNA. The modified base pairs are rendered as CPK spheres, with εA, 3d3mA and THF in green and the opposing thymidine in magenta. (D) Side view of the extrahelical THF-T bulge trapped by AlkD (blue).
N3- and N7-alkylated purine nucleobases are highly detrimental to the cell.22,23 3mA and a ring-opened formamidopyrimidyl derivative of 7mG, 2,6-diamino-4-hydroxy-N5-(methyl)-formamidopyrimidine, are cytotoxic by virtue of their ability to inhibit DNA synthesis.24–26 As a consequence of their positively charged purine rings, 3mA and 7mG are highly susceptible to spontaneous depurination, leading to formation of abasic sites that are both cytotoxic and mutagenic.27–29 Thus, glycosylase excision of cationic 3mA and 7mG does not require activation by a general acid or a substantial amount of catalytic power 6. Interestingly, most 3mA-specific glycosylases retain excision activity in the absence of specific catalytic residues, suggesting that direct side chain chemistry does not fully account for the observed rate enhancements by these enzymes.30–34 Because spontaneous depurination rates of N7-alkylguanines depend on the DNA secondary structural context,14,35–37 it is reasonable to postulate that the specific DNA conformation in the vicinity of the lesion contributes to excision of these adducts, although this idea has not been explored in any detail.
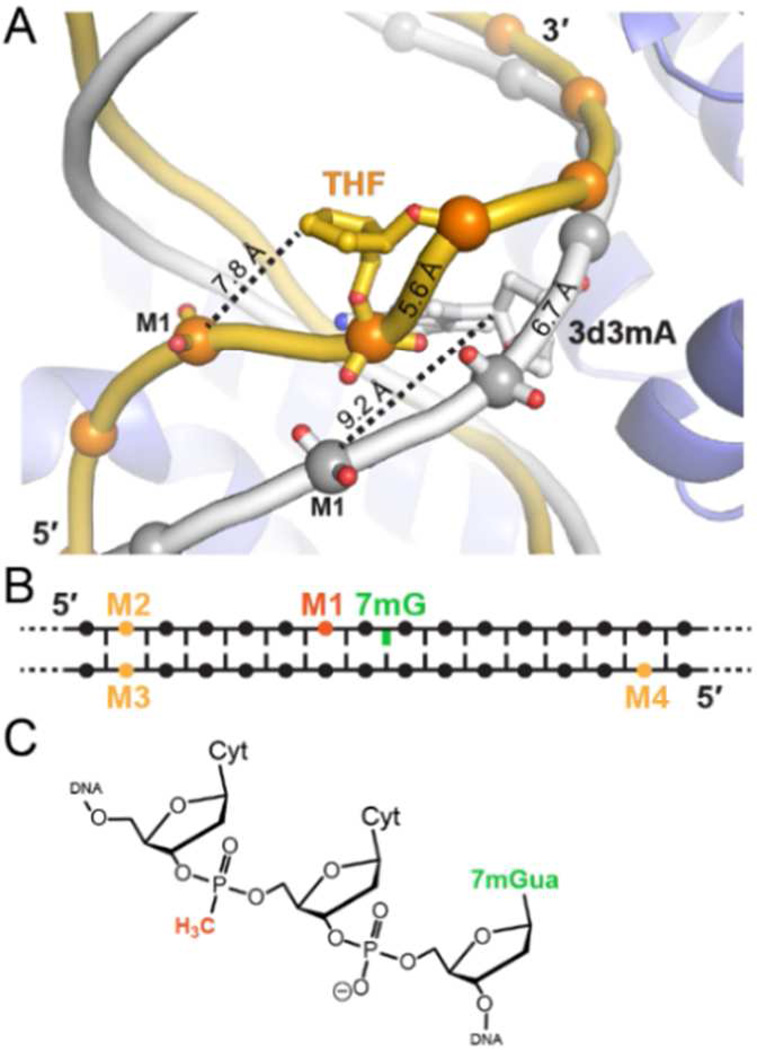
We recently determined several crystal structures of a unique 3mA/7mG DNA glycosylase, AlkD, in complex with alkylpurine, mismatched, and abasic DNA, all of which exhibit the same general protein-DNA binding regime.34,37–40 AlkD does not flip the lesion into a binding pocket, but instead binds the undamaged DNA strand and positions the lesion on the opposite face of the DNA helix from the protein binding surface (Figure 1B, C). Most strikingly, there are no contacts between the protein and the lesion. The alkylpurine and mismatched base pairs are highly sheared but remain stacked in the duplex, whereas the abasic site and its opposite nucleotide are rotated out of the helix to create a 1-nucleotide bubble with the flanking base pairs stacked (Figure 1D). This distortion to the DNA backbone positions the flipped ribose ring in closer proximity to the phosphate of the nucleotide immediately 5′ to the lesion (position M1, Figure 2). The distance between this M1 phosphate and the C1′ of the flipped nucleotide is 20% shorter than in the un-flipped AlkD alkylpurine/mismatch complexes and in normal B-DNA (Figure S3).
Figure 2.
(A) Overlay of AlkD THF-DNA (gold) and 3d3mA-DNA (gray) complexes. The phosphate 5′ to the lesion is designated M1. Distances between M1 and the C1′ carbon of the lesion are marked with dashed lines, and distances between the phosphates covalently attached to the lesion are shown on the backbone. (B) Schematic showing the relative positions of 7mG and MeP substitutions used in this study. (C) Chemical structure of MeP in the M1 sequence context.
The skewed DNA conformation and the absence of protein contacts to the lesion in the AlkD-DNA complex led us to hypothesize that the phosphate backbone plays a substantial role in 7mG depurination. To test this, we measured the rates of AlkD-catalyzed and spontaneous 7mG release from oligo-nucleotides containing non-bridging methylphosphonate (MeP) substitutions at various phosphate positions (Figure 2B). MeP eliminates the negative charge at that position (Figure 2C) and was a key strategy in determining the importance of phosphates to UDG catalysis.16,17 We introduced a MeP at the proximal nucleotide 5′ to the 7mG (M1), and as controls for general effects of MeP substitution, at three sites located 6 nucleotides from the lesion and outside of the protein binding region on both damaged (M2) and undamaged (M3, M4) strands (Figure 2B).
Compared to the substrate containing no MeP (M0), the control sequences M2 and M3 had no effect on either the AlkD single-turnover (kst) or non-enzymatic (knon) rates of 7mG depurination, and M4 had only a modest (3-fold) effect on kst (Table 1, Figure S4). In contrast, the M1 substitution resulted in a 15-fold reduction in AlkD activity and a 30-fold (96%) reduction in rate enhancement (kst/knon) compared to M0 (Table 1). Thus, excision of 7mG by AlkD is largely dependent on the conformation of the phosphate backbone in the vicinity of the lesion. Interestingly, the modest increase in knon for M1-MeP is similar to that of 7mG depurination from single-stranded DNA (2.8 × 10−6 s−1) 37, suggesting that the MeP substitution perturbs the secondary structure of the unbound DNA. Regardless of this effect, the larger effect of MeP substitution on the AlkD catalyzed rate versus the spontaneous rate of 7mG depurination indicates that the 96% reduction in M1/M0 rate enhancement is attributed to the specific enzyme-DNA complex and not by an effect on the DNA alone.
Table 1.
Rates of AlkD-catalyzed and spontaneous 7mG depurination from MeP-DNA
| kst (× 10−3 s−1) a | knon (× 10−6 s−1) a | kst / knon | |
|---|---|---|---|
| M0 | 23.6 ± 1.1 | 1.0 ± 0.1 | 2.4 × 104 |
| M1 | 1.6 ± 0.4 | 1.8 ± 0.2 | 8.9 × 102 |
| M2 | 23.6 ± 1.1 | 1.0 ± 0.1 | 2.4 × 104 |
| M3 | 23.2 ± 1.2 | 1.1 ± 0.03 | 2.1 × 104 |
| M4 | 7.7 ± 0.5 | 1.1 ± 0.1 | 7.0 × 103 |
Single-turnover (kst) and non-enzymatic (knon) rate constants for 7mG depurination from 25-mer oligonucleotides at 37°C, pH 7.5, and 150 mM ionic strength. M1-M4 each contained one MeP at the positions shown in Figure 2B. Data for M0 (no MeP) were taken from ref.37 DNA sequences and kinetic data can be found in the Supplement.
The residual 7mG depurination activity in the M1 substrate may stem from the phosphates immediately 5′ and 3′ to the lesion, which reside 5 Å from the lesion C1′ in the flipped THF complex (Figure S3). However, we were unable to substitute MeP at these positions due to the manner in which the 7mG substrate is prepared (see Supporting Information). Although these flanking phosphates are closer to the lesion than M1, only M1 moves closer to the lesion upon base flipping (Figure S3). The modest reduction in 7mG excision from the M4 substrate is consistent with preferential binding of the enzyme to the unmodified strand, and may reflect a preference of the enzyme to initially bind the 5′ ends of an oligo-nucleotide.
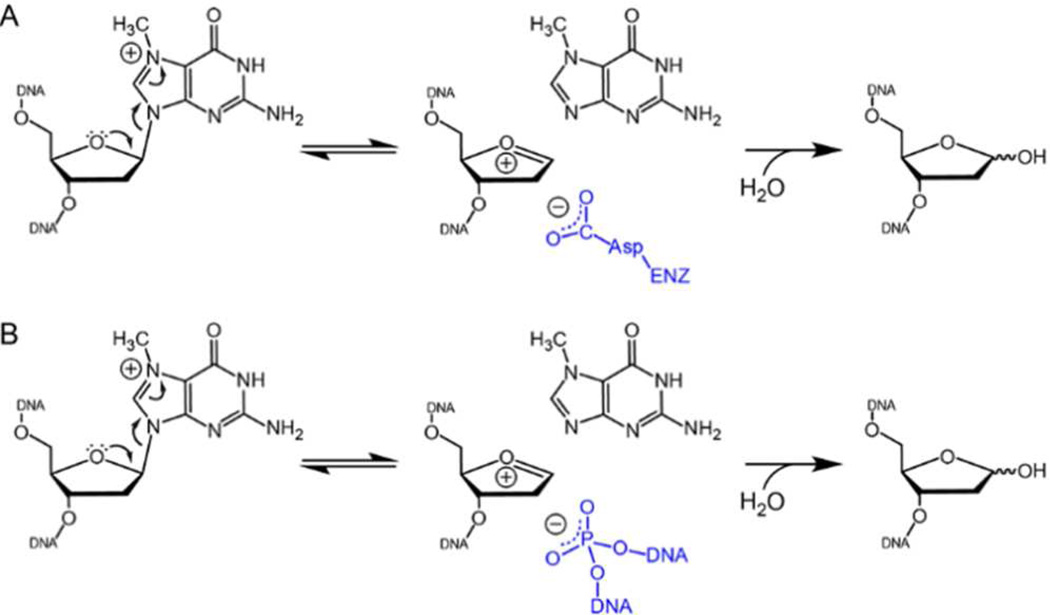
Given the absence of protein functional groups in the vicinity of the lesion, we conclude that catalysis of 7mG excision by AlkD is driven by the DNA, and speculate that the proximal phosphates facilitate depurination by either stabilizing the oxocarbenium intermediate or positioning the attacking water. Introduction of MeP, which eliminates the negative charge and replaces a polar oxygen with an aliphatic methyl group, could have effected either the electrostatic environment or the specific conformation of the DNA. The reduction in distance from the M1 phosphate to the lesion upon base flipping suggests that the observed effect of MeP substitution may have disrupted a stabilizing electrostatic interaction between the anionic phosphate backbone and the oxocarbenium (Figure 3). For this to be true, however, the M1 phosphate would need to be closer to the lesion than the 7.8 Å observed in the THF structure, and it is not unreasonable to postulate that a cationic 3mA or 7mG substrate would have such an additional distortive effect. Alternatively, the phosphate group may stabilize a particular DNA conformation necessary for catalysis. Indeed, the nucleic acid secondary structure is the basis for designed DNAs (DNAzymes) that catalyze various metal-dependent RNA cleavage and DNA ligation reactions.41,42 In addition to a direct interaction, the phosphate could deprotonate or stabilize any developing positive charge on the attacking water. Regardless of the specific mechanism employed, this work, together with the crystal structures, establishes that the specific conformation of the DNA backbone captured by the enzyme positions the phosphate for catalysis. To our knowledge, this is the first example of an active site composed exclusively of DNA atoms.
Figure 3.
Protein and/or DNA functional groups provide an anionic environment to drive base excision. The chemical mechanism of 7mG depurination is shown, with stabilization of the oxocarbenium reaction intermediate by (A) a conserved carboxylate side chain and (B) a DNA phosphate moiety.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by a grant from the National Science Foundation (MCB-1122098). Additional support for facilities was provided by the Vanderbilt Center in Molecular Toxicology (P30 ES000267) and the Vanderbilt-Ingram Cancer Center (P30 CA068485). E.H.R. was supported by the Vanderbilt Training Program in Environmental Toxicology (NIH T32 ES07028).
Footnotes
ASSOCIATED CONTENT
Supporting Information
Experimental procedures and representative kinetic data. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
REFERENCES
- 1.Brooks SC, Adhikary S, Rubinson EH, Eichman BF. Biochim Biophys Acta. 2013;1834:247–271. doi: 10.1016/j.bbapap.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werner RM, Stivers JT. Biochemistry. 2000;39:14054–14064. doi: 10.1021/bi0018178. [DOI] [PubMed] [Google Scholar]
- 3.Chen XY, Berti PJ, Schramm VL. J Am Chem Soc. 2000;122:1609–1617. [Google Scholar]
- 4.Dinner AR, Blackburn GM, Karplus M. Nature. 2001;413:752–755. doi: 10.1038/35099587. [DOI] [PubMed] [Google Scholar]
- 5.McCann JA, Berti PJ. J Am Chem Soc. 2008;130:5789–5797. doi: 10.1021/ja711363s. [DOI] [PubMed] [Google Scholar]
- 6.Stivers JT, Jiang YL. Chem Rev. 2003;103:2729–2759. doi: 10.1021/cr010219b. [DOI] [PubMed] [Google Scholar]
- 7.Berti PJ, McCann JA. Chem Rev. 2006;106:506–555. doi: 10.1021/cr040461t. [DOI] [PubMed] [Google Scholar]
- 8.Drohat AC, Jagadeesh J, Ferguson E, Stivers JT. Biochemistry. 1999;38:11866–11875. doi: 10.1021/bi9910878. [DOI] [PubMed] [Google Scholar]
- 9.Labahn J, Scharer OD, Long A, Ezaz-Nikpay K, Verdine GL, Ellenberger TE. Cell. 1996;86:321–329. doi: 10.1016/s0092-8674(00)80103-8. [DOI] [PubMed] [Google Scholar]
- 10.Hollis T, Ichikawa Y, Ellenberger T. Embo J. 2000;19:758–766. doi: 10.1093/emboj/19.4.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norman DP, Chung SJ, Verdine GL. Biochemistry. 2003;42:1564–1572. doi: 10.1021/bi026823d. [DOI] [PubMed] [Google Scholar]
- 12.Brinkmeyer MK, Pope MA, David SS. Chem Biol. 2012;19:276–286. doi: 10.1016/j.chembiol.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen XY, Berti PJ, Schramm VL. J Am Chem Soc. 2000;122:6527–6534. [Google Scholar]
- 14.Gates KS, Nooner T, Dutta S. Chem Res Toxicol. 2004;17:839–856. doi: 10.1021/tx049965c. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien PJ, Ellenberger T. Biochemistry. 2003;42:12418–12429. doi: 10.1021/bi035177v. [DOI] [PubMed] [Google Scholar]
- 16.Jiang YL, Ichikawa Y, Song F, Stivers JT. Biochemistry. 2003;42:1922–1929. doi: 10.1021/bi027014x. [DOI] [PubMed] [Google Scholar]
- 17.Parker JB, Stivers JT. Biochemistry. 2008;47:8614–8622. doi: 10.1021/bi800854g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogacheva MV, Saparbaev MK, Afanasov IM, Kuznetsova SA. Biochimie. 2005;87:1079–1088. doi: 10.1016/j.biochi.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Jiang YL, Stivers JT. Biochemistry. 2001;40:7710–7719. doi: 10.1021/bi010622c. [DOI] [PubMed] [Google Scholar]
- 20.Werner RM, Jiang YL, Gordley RG, Jagadeesh GJ, Ladner JE, Xiao G, Tordova M, Gilliland GL, Stivers JT. Biochemistry. 2000;39:12585–12594. doi: 10.1021/bi001532v. [DOI] [PubMed] [Google Scholar]
- 21.Bianchet MA, Seiple LA, Jiang YL, Ichikawa Y, Amzel LM, Stivers JT. Biochemistry. 2003;42:12455–12460. doi: 10.1021/bi035372+. [DOI] [PubMed] [Google Scholar]
- 22.Beranek DT. Mutat Res. 1990;231:11–30. doi: 10.1016/0027-5107(90)90173-2. [DOI] [PubMed] [Google Scholar]
- 23.Gates KS. Chem Res Toxicol. 2009;22:1747–1760. doi: 10.1021/tx900242k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boiteux S, Huisman O, Laval J. Embo J. 1984;3:2569–2573. doi: 10.1002/j.1460-2075.1984.tb02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larson K, Sahm J, Shenkar R, Strauss B. Mutat Res. 1985;150:77–84. doi: 10.1016/0027-5107(85)90103-4. [DOI] [PubMed] [Google Scholar]
- 26.O'Connor TR, Boiteux S, Laval J. Nucleic Acids Res. 1988;16:5879–5894. doi: 10.1093/nar/16.13.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence CW, Borden A, Banerjee SK, LeClerc JE. Nucleic Acids Res. 1990;18:2153–2157. doi: 10.1093/nar/18.8.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loeb LA, Preston BD. Annu Rev Genet. 1986;20:201–230. doi: 10.1146/annurev.ge.20.120186.001221. [DOI] [PubMed] [Google Scholar]
- 29.Xiao W, Samson L. Proc Natl Acad Sci U S A. 1993;90:2117–2121. doi: 10.1073/pnas.90.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drohat AC, Kwon K, Krosky DJ, Stivers JT. Nat Struct Biol. 2002;9:659–664. doi: 10.1038/nsb829. [DOI] [PubMed] [Google Scholar]
- 31.Cao C, Kwon K, Jiang YL, Drohat AC, Stivers JT. J Biol Chem. 2003;278:48012–48020. doi: 10.1074/jbc.M307500200. [DOI] [PubMed] [Google Scholar]
- 32.Eichman BF, O'Rourke EJ, Radicella JP, Ellenberger T. Embo J. 2003;22:4898–4909. doi: 10.1093/emboj/cdg505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metz AH, Hollis T, Eichman BF. Embo J. 2007;26:2411–2420. doi: 10.1038/sj.emboj.7601649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubinson EH, Metz AH, O'Quin J, Eichman BF. J Mol Biol. 2008;381:13–23. doi: 10.1016/j.jmb.2008.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vodicka P, Hemminki K. Chem Biol Interact. 1988;68:117–126. doi: 10.1016/0009-2797(88)90010-5. [DOI] [PubMed] [Google Scholar]
- 36.Lindahl T, Nyberg B. Biochemistry. 1972;11:3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- 37.Rubinson EH, Gowda AS, Spratt TE, Gold B, Eichman BF. Nature. 2010;468:406–411. doi: 10.1038/nature09428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubinson EH, Eichman BF. Current opinion in structural biology. 2012;22:101–109. doi: 10.1016/j.sbi.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alseth I, Rognes T, Lindback T, Solberg I, Robertsen K, Kristiansen KI, Mainieri D, Lillehagen L, Kolsto AB, Bjoras M. Mol Microbiol. 2006;59:1602–1609. doi: 10.1111/j.1365-2958.2006.05044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dalhus B, Helle IH, Backe PH, Alseth I, Rognes T, Bjoras M, Laerdahl JK. Nucleic Acids Res. 2007;35:2451–2459. doi: 10.1093/nar/gkm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breaker RR. Curr Opin Chem Biol. 1997;1:26–31. doi: 10.1016/s1367-5931(97)80105-6. [DOI] [PubMed] [Google Scholar]
- 42.Breaker RR. Nat Biotechnol. 1997;15:427–431. doi: 10.1038/nbt0597-427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.