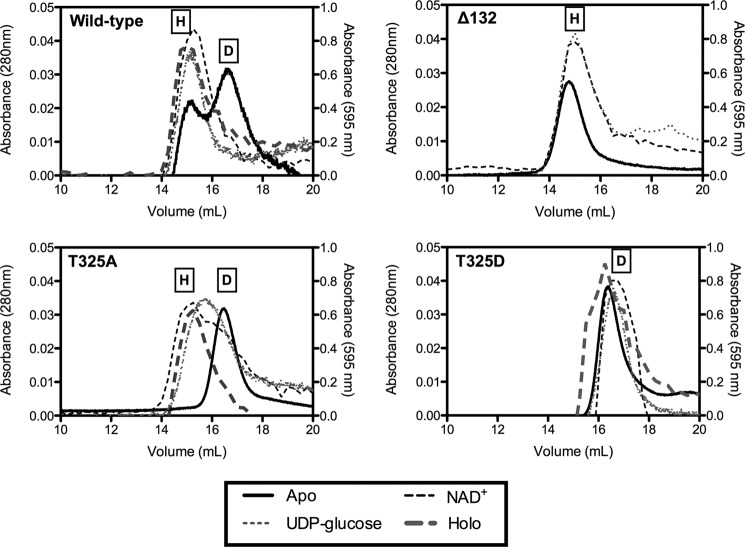
FIGURE 4.
Quaternary structure of wild-type UGDH and T325A is stabilized from dimeric to hexameric by substrate and cofactor, whereas T325D remains an obligatory dimer. Purified wild-type and mutant UGDH was fractionated by gel filtration FPLC in the absence (dark solid line) or presence of NAD+ alone (gray hatched line), UDP-glucose alone (gray dotted line), or both (dark hatched line). Absorbance was monitored at 280 nm for the apoproteins (left ordinate axis) and 595 nm for the holoproteins (right ordinate axis). Size determination of each peak was made by comparison to molecular weight standards. Representative traces are plotted for wild-type UGDH, Δ132, T325A, and T325D. On each plot the peaks corresponding to hexamer and dimer are labeled H and D, respectively.