FIGURE 1.
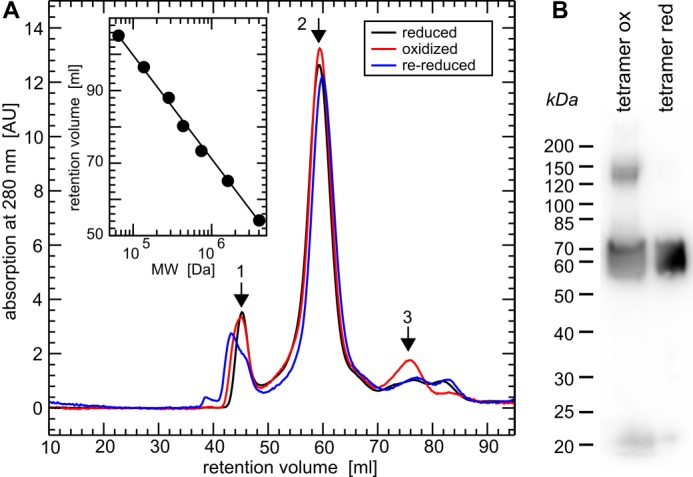
Quaternary structure of reduced, oxidized, and re-reduced CRMP2. A, gel filtration chromatography (HiLoad 16/60 Superdex 200 column, GE Healthcare) of (Grx2c+DTT+TCEP) reduced, H2O2-oxidized, and re-reduced recombinant CRMP2 in their native, folded states. Calibration (inset) was done using the manufacturer's instructions: 1, ∼800 kDa, i.e. dodecamer (theoretical mass, 770.4 kDa); 2, 256 kDa, i.e. (homo)tetramer (theoretical mass, 256.8 kDa); 3, 65 kDa, i.e. monomeric CRMP2 (theoretical mass, 64.2 kDa). B, non-reducing SDS-PAGE and Western blot of the tetrameric fractions of oxidized and reduced CRMP2 following the gel-filtration chromatography depicted in A and denaturation of the protein complexes.
