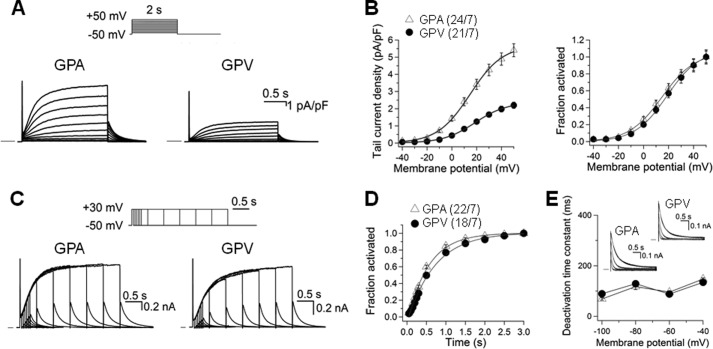
FIGURE 6.
Comparison of IKs current density and gating kinetics in guinea pig atrial and ventricular myocytes. Whole cell currents recorded in sodium-, potassium-, and calcium-free solution at 35 °C in the presence of 3 μm E4031. A, representative current traces elicited by the diagrammed voltage clamp protocol applied once per 10 s. Currents were normalized by cell capacitance (calibrations applied to both panels). Horizontal bars denote zero current. pA, picoampere; pF, picofarad. B, left panel, tail current density plotted against test pulse voltage. Right panel, voltage-dependence of IKs activation. For each myocyte, the relationship between tail current amplitude (Itail) and test pulse voltage (Vt) was fit with a simple Boltzmann function as described in Fig. 3A. The fraction of channel activated (Itail/Imax) was plotted against Vt. C, current traces elicited by an “envelope test” protocol. Test pulse durations ranged from 50–3000 ms, and the interpulse interval was 10 s. nA, nanoampere. D, time course of IKs activation constructed from the growth of tail currents during the envelope test protocol as shown in C. For each myocyte, the relationship between tail current amplitude (Itail) and test pulse duration (t) was fit with a single exponential function with a delay, d: Itail = Iss(1-exp[-(t-d)/τ]), where Iss and τ are the plateau Itail amplitude and time constant of IKs activation. E, rate of IKs deactivation. Inset, representative IKs tail currents from atrial and ventricular myocytes recorded at repolarization voltages (Vr) of −100 to −40 mV in 20-mV increments. The time courses were fit with a double (−40 and −60 mV) or single (-80 and −100 mV) exponential function: Itail = Σ Iiexp(-t/τd), where i = 1 or 2 and τd was the deactivation time constant of component i. The τd values (of the major component, for double exponential fit) were pooled and plotted against repolarization voltages. In B, left panel, and D, the numbers in parentheses denote the number of myocytes studied/number of animals used.