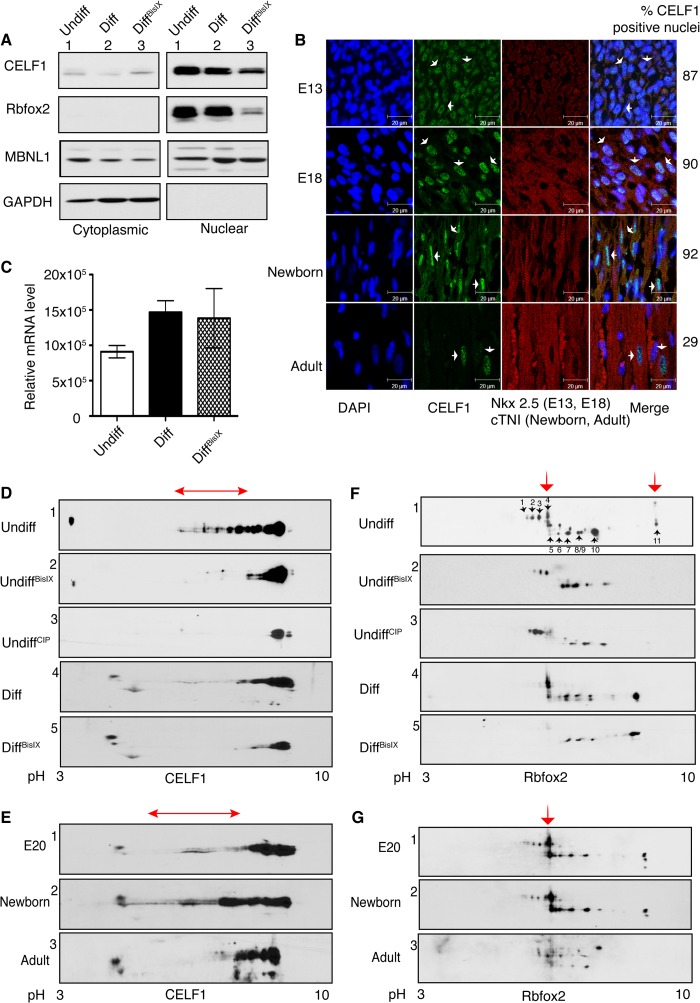
FIGURE 5.
Inhibition of PKC activity reduces the phosphorylation and steady state levels of CELF1 and Rbfox2. A, WB analysis of nuclear and cytoplasmic fractions from undiff, mock-treated (Diff), and PKC inhibitor-treated differentiated (DiffBisIX) H9c2 cells using antibodies against CELF1, Rbfox2, MBNL1, and GAPDH. B, IHC of sagittal rat heart sections using antibodies against CELF1, Nkx2.5 (cardiomyocyte marker for E13 and E18), and cTNI (cardiomyocyte marker for newborn and adult). Nuclei are stained with DAPI. Cardiomyocytes that are CELF1-positive were quantified counting >150 nuclei at each developmental stage. Arrows indicate representative cells. C, relative Rbfox2 mRNA levels was determined by semi-quantitative RT-PCR using RNA from H9c2 cells (Undiff, Diff, and DiffBisIX) by net intensity calculation of the PCR product using Kodak gel Logic 2200. D and F, two-dimensional/Western blot analysis of nuclear fractions from undiff, undiffCIP (calf intestinal phosphatase-treated), diff, and diffBisIX H9c2 cells using anti-CELF1 (D) or anti-Rbfox2 (F) Abs. The red arrows indicate PKC-dependent phospho-isoforms. E and G, protein lysates from E20, newborn, and adult (6-month old) rat hearts were separated on two-dimensional gels followed by WB using anti-CELF1 (E) and anti-Rbfox2 (G) antibodies. The red arrows indicate PKC-induced phosphorylated isoforms. E20 and newborn hearts were pooled, n = 4. Results are representative of at least three independent experiments.