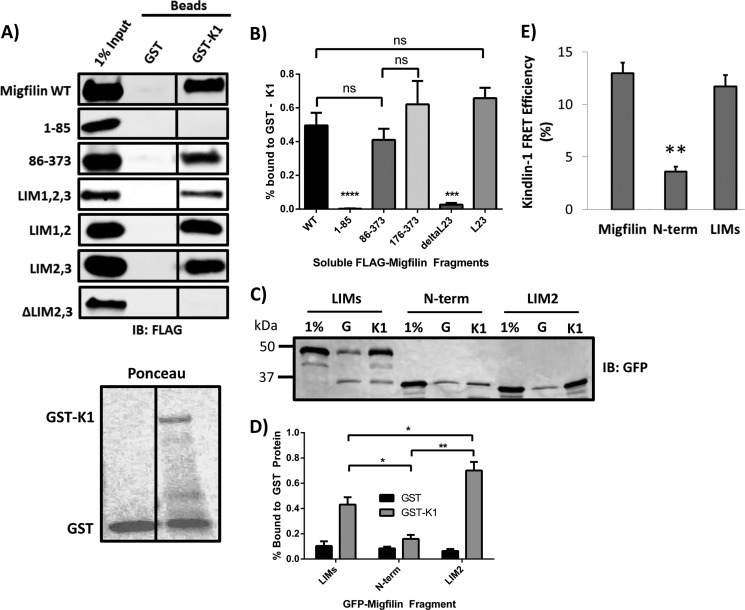
FIGURE 6.
Migfilin interaction with GST-kindlin-1 requires LIM domains. A, lysates of CHO cells overexpressing FLAG-migfilin constructs were incubated with beads coated with either GST or GST-kindlin-1 (GST-K1). Beads were washed, bound proteins eluted with SDS-sample buffer and fractionated by SDS-PAGE. Bound migfilin was detected by Western blotting with anti-FLAG antibodies. Migfilin proteins containing the LIM domains (WT, 86–373, LIMs, LIM1,2, and LIM2,3) pull down with GST-kindlin-1, whereas 1–85 and ΔLIM2,3 do not. Black vertical lines between Western blot lanes denote non-adjacent lanes on the same blot, with the same level of exposure and contrast. Lower panel, Ponceau stain of the membrane shows loading of the GST and GST-kindlin-1 affinity matrices. B, kindlin-1 binding quantification of constructs with n ≥ 5. ****, p < 0.0001; ***, p < 0.001 using Student's t test. C, GFP-migfilin constructs were tested in a GST pulldown assay and quantification. D shows that LIMs and LIM2 bind GST-K1 above that of the migfilin N-terminal negative control; n = 3; *, p < 0.05; **, p < 0.01. E, fibroblasts on fibronectin coexpressed GFP-migfilin constructs and mCherry-kindlin-1 and FRET efficiency was quantified using FLIM; **, p < 0.001 using Student's t test.