Abstract
It is well established that animals including humans attribute greater reinforcing value to glucose-containing sugars compared to their non-caloric counterparts, generally termed ‘artificial sweeteners’. However, much remains to be determined regarding the physiological signals and brain systems mediating the attribution of greater reinforcing value to sweet solutions that contain glucose. Here we show that disruption of glucose utilization in mice produces an enduring inhibitory effect on artificial sweetener intake, an effect that did not depend on sweetness perception or aversion. Indeed, such an effect was not observed in mice presented with a less palatable, yet caloric, glucose solution. Consistently, hungry mice shifted their preferences away from artificial sweeteners and in favour of glucose after experiencing glucose in a hungry state. Glucose intake was found to produce significantly greater levels of dopamine efflux compared to artificial sweetener in dorsal striatum, whereas disrupting glucose oxidation suppressed dorsal striatum dopamine efflux. Conversely, inhibiting striatal dopamine receptor signalling during glucose intake in sweet-naïve animals resulted in reduced, artificial sweetener-like intake of glucose during subsequent gluco-deprivation. Our results demonstrate that glucose oxidation controls intake levels of sweet tastants by modulating extracellular dopamine levels in dorsal striatum, and suggest that glucose utilization is one critical physiological signal involved in the control of goal-directed sweetener intake.
Key points
Much remains to be determined regarding the physiological signals and brain systems that mediate the attribution of greater reward to sugars compared to artificial sweeteners.
We show that disruption of glucose utilization in mice produces an enduring inhibitory effect on artificial sweetener intake.
Consistently, hungry mice shifted their preferences away from artificial sweeteners and in favour of glucose after experiencing glucose in a hungry state.
Disrupting glucose oxidation suppressed dorsal striatum dopamine efflux during sugar intake.
Glucose oxidation controls intake levels of sweet tastants by modulating extracellular dopamine levels in dorsal striatum.
Introduction
Sweet soft drinks are a major source of excessive ingestion of sugar-derived calories (Bray et al. 2004). Intriguingly, the introduction of artificially sweetened drinks into the market was not sufficient to curb overall sugar intake (Saris 2003). In fact, accumulating evidence reveals that the glucose moiety of sweet carbohydrates has the ability to activate taste-independent physiological pathways to stimulate sugar intake. Thus, gut infusions of glucose-containing sugars performed in response to oral ingestion of a distinct flavour result in enduring preferences for that particular flavour, as shown by ‘flavour-nutrient conditioning’ paradigms in rodents (Sclafani, 2001; Mobini et al. 2007). Flavour preference learning has also been shown to be relevant for human behaviour, as post-ingestive effects also modulate flavour preference in healthy adults (Mobini et al. 2007; Yeomans et al. 2009). Consistently, sweet-insensitive mutant mice lacking the taste ion channel TRPM5 are capable of acquiring preferences for sipper positions associated with sugar intake (de Araujo et al. 2008), resulting in ingested levels comparable to those observed in sweet-sensitive wild-type mice (Ren et al. 2010). However, and despite the relevance of the topic to our understanding of human eating habits, a full characterization of the physiological pathways that bring about increased sweet-independent sugar preferences is currently lacking.
Also incompletely understood are the brain systems that mediate such flavour-independent stimulation of sugar intake. While the central catecholamine transmitter dopamine seemingly plays a critical role in mediating these responses (de Araujo et al. 2008, 2010; Sclafani et al. 2011), it remains unclear which of the dopaminergic targets constitute the critical regulator of sweet-independent sugar intake. At this point we stress the central role played by the dorsal aspect of the striatum in food reinforcement. First, genetic ablation of dopamine synthesis induces marked hypophagia, an effect reversed by restoring dopamine signalling in dorsomedial striatum (Szczypka et al. 1999; Sotak et al. 2005). Furthermore, several studies show that the integrity of mediodorsal striatal circuits is critical for the formation of action–outcome (e.g. lever press–food) associations during instrumental tasks (Taylor & Robbins, 1986; Yin et al. 2005, 2008; Balleine et al. 2009). Specifically, it is assumed that dopamine signalling in dorsal striatal circuits acts as one critical determinant of whether animals will display devaluation-sensitive (‘goal-directed’) or devaluation-insensitive (‘habitual’) ingestive behaviours (Yin et al. 2008).
To gain further insight on the role of cellular utilization of glucose moieties in sweet-independent attribution of value to sugars, we tested the hypothesis that glucose oxidation rates regulate both behavioural output and mediodorsal striatum dopamine efflux during active sweet intake. To test our hypothesis, we obtained concomitant behavioural and neurochemical measurements during the active ingestion of sugars and non-caloric artificial sweeteners, such that glucose oxidation rates were pharmacologically controlled during either of these behavioural contexts. We predicted that disrupting glucose oxidation would produce persistent decreases in intake that are specific to artificial sweeteners, while glucose ingestion would remain immune to such manipulations.
Methods
Subjects
In total, 138 wild-type adult male mice on a C57BL6/J background were used. At the time of experiments animals were 8–16 weeks old. All experiments were conducted in accordance with the J.B. Pierce Laboratory and Yale University regulations on usage of animals in research.
Surgical procedures for implantation of gastric catheters and microdialysis guiding cannulae
Once animals had been anaesthetized with an i.p. injection of a ketamine/xylazine (100/15 mg kg−1), a midline incision was made into the abdomen. The stomach was exteriorized through the midline incision and a purse string suture was placed in its non-glandular region, into which the tip of MicroRenathane tubing (Braintree Scientific Inc., Braintree, MA, USA) was inserted. The purse string was tightened around the tubing, which was then tunnelled subcutaneously to the dorsum via a small hole made into the abdominal muscle; a small incision to the dorsum between the shoulder plates was then made to allow for catheter exteriorization. Incisions were sutured and thoroughly disinfected and the exterior end of the catheter was plugged. For animals used in the microdialysis experiments targeting the dorsal striatal region, the animal was placed on a stereotaxic apparatus (David Kopf, Tujunga, CA, USA) under constant flow of ∼1% isoflurane anaesthesia (1.5 l min−1) and a circular craniotomy was drilled at AP = 1.3 mm and ML =±1.3 mm implantation of a guide cannulae (DV =−0.5 mm from brain surface) for posterior insertion of a microdialysis probe (final probe tip positions (DV =−2.5 mm from brain surface)].
Stimuli and behavioural apparatus
Both taste stimuli (the carbohydrate glucose and the artificial sweetener sucralose) were obtained from Sigma (St Louis, MO, USA) and prepared daily (at 0.8 and 2 mm, respectively) in distilled water at room temperature. Behavioural experiments were conducted in either one of three identical mouse behaviour chambers enclosed in a ventilated and sound-attenuating cubicle (Med Associates Inc., St. Albans, VT, USA). Each chamber was equipped with two slots for sipper tubing placements, at symmetrical locations on one of the cage walls. All sippers were connected to a contact-based lick detection device allowing for measurements of licking responses with 10 ms resolution. All lick timestamps were saved in a computer file for posterior analysis.
Short-term two-bottle preference tests
Short-term (5 min) two-bottle preference tests between d-glucose and the artificial sweetener sucralose were used to determine the short-term, oral relative preferences for each of these compounds. The short duration of this test aims to minimize post-ingestive influences. Once habituated to the behavioural chamber and the stimuli for 4 days, each animal was presented with the choice between d-glucose and sucralose. The number of licks in each sipper was recorded and used to calculate the preference ratio as follows:
where n(Sipperx) denotes the detected number of licks to sipper x during a given session. To eliminate the influence of side-biases, mice were tested for four consecutive days with sipper positions being switched daily.
Indirect calorimetry
Energy expenditure was measured via indirect calorimetry using the Oxymax/CLAMS Animal Monitoring System (Columbus Instruments, Columbus, OH, USA). This is a mouse-dedicated, four-cage system equipped with open-circuit calorimetry, contact lickometers and XZ-axis motor activity sensors. Metabolism-induced heat was derived by assessing the exchange of oxygen for carbon dioxide that occurs during metabolic processes (Jequier et al. 1987), as measured by the mass flow principle. Oxygen (O2) measurement was performed via paramagnetic sensing and carbon dioxide (CO2) by single beam non-dispersed IR. The respective volumes were determined as:
| (1) |
| (2) |
where Vi is the mass of air at chamber input per unit time, Vo is the mass of air at chamber output per unit time, O2i is the oxygen fraction in Vi,CO2i is the carbon dioxide fraction in Vi, O2o is the oxygen fraction in Vo and CO2o is the carbon dioxide fraction in Vo.
The respiratory quotient RQ was calculated as
| (3) |
Heat was calculated by determining the calorific value of the food being metabolized. For the accepted range of nutritional RQs (0.707–1.0), the heat available is 4.686–5.047 kcal (l O2)−1. The calorific value (Cv) is interpolated by straight line approximation for values within the RQ range (Cv= 3.815 + 1.232 × RQ). The resulting calorific value is applied to the obtained figure for oxygen consumption for derivation of heat, followed by normalization of this quantity to the animal's body volume:
| (4) |
Ambulatory activity was obtained from the total number of beam-break counts of the XZ sensors.
Longer-term (1 h) intake task
Prior to obtaining the final behavioural test, animals were habituated to the behavioural boxes and sweet stimuli during four daily 1 h sessions. These sessions were preceded by i.p. injections of either vehicle (saline) or of the glucose anti-metabolite 2-deoxy-d-glucose (2-DG; Sigma, 400 mg kg−1) 10 min prior to the beginning of the session. Animals were randomly assigned to one of four experimental groups: Group 1, ‘Naive glucose’ group, treated with vehicle injections 10 min prior to each of the four habituation sessions, where the animal is allowed to freely consume 0.8 m glucose solutions; Group 2, ‘Naive sucralose’ group, treated with vehicle injections 10 min prior to each of the habituation sessions, where the animal is allowed to freely consume 2 mm sucralose solutions; Group 3, ‘Recurrent glucose’ group, treated with either vehicle or 2-DG injections on alternate days 10 min prior to each of the four habituation sessions, where the animal is allowed to freely consume 0.8 m glucose solutions; and Group 4, ‘Recurrent sucralose’ group, treated with either vehicle or 2-DG injections on alternate days 10 min prior to each of the habituation sessions, where the animal is allowed to freely consume 2 mm sucralose solutions. The final behavioural test measures were obtained on session days 5 and 6, when all animals were treated with vehicle (day 5) or 2-DG (day 6) 10 min prior to ingesting glucose (groups 1 and 3) or sucralose (groups 2 and 4). These experimental groups are shown in schematic form in Table 1. Lick timestamps were analysed posteriorly and lick counts were binned every 10 min as arbitrarily specified a priori. During sessions 5 and 6 microdialysis samples were collected concomitantly to behavioural performance (see below). Two additional control groups injected for 6 days with vehicle only were presented with either glucose or sucralose as above.
Table 1.
Experimental design for each of the tested groups
| Pre-treatment (i.p. injection 10 min prior to behaviour) | ||||||
|---|---|---|---|---|---|---|
| Habituation Sessions (1 h behavioural assay) | Test sessions (1 h behavioural assay combined with microdialysis in groups 1–4) | |||||
| Group name (treatment/sweetener) | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 |
| Naïve glucose | VEH | VEH | VEH | VEH | VEH | 2-DG |
| Naïve sucralose | VEH | VEH | VEH | VEH | VEH | 2-DG |
| Recurrent glucose | VEH | 2-DG | VEH | 2-DG | VEH | 2-DG |
| Recurrent sucralose | VEH | 2-DG | VEH | 2-DG | VEH | 2-DG |
| Control glucose | VEH | VEH | VEH | VEH | VEH | VEH |
| Control sucralose | VEH | VEH | VEH | VEH | VEH | VEH |
Conditioned taste aversion assessment
To rule out the possibility that the inhibitory effect of 2-DG on artificial sweetener intake was due to conditioned aversion to sweetener taste, we compared the ability of a malaise-inducing agent [0.35 m lithium chloride, 10 μl (g body weight)−1] to induce taste aversion in a classical conditioned taste aversion paradigm versus in the experimental design similar to the one described above. One group of animals was treated with 2-DG 10 min prior to the beginning of 10-min two-bottle sucralose versus water preference tests. On the following day the same preference test was performed in the absence of drug treatment. Then, a second group of animals was exposed to the same protocol except that 0.35 m lithium chloride was used instead of 2-DG. Importantly, these two groups of non-naïve animals had been exposed to sucralose versus water tests prior to the treatment day, in analogy to our main behavioural protocol. Finally, a third group of sweet-naïve animals was first exposed to the 10 min two-bottle sucralose versus water preference test and then treated with 0.35 m lithium chloride (as in canonical conditioned taste aversion paradigms). A preference test was performed on the following day as for the other groups. Preference ratios were computed as explained above.
Dopamine measurements during behavioural performance in the 1 h intake task
During the test sessions (i.e. session days 5 and 6) for the 1 h task described above, collection of microdialysate samples from the mediodorsal striatum was performed concomitant with behavioural performance. An additional group of naïve animals was exposed to sucralose tests as above but treated with dichloroacetate (400 mg kg−1, Sigma), a compound that promotes glucose oxidation, instead of 2-DG. Specifically, during the experimental sessions, microdialysate samples from these freely moving mice were collected, separated and quantified by HPLC coupled to electro-chemical detection methods (‘HPLC-ECD’). Briefly, after recovery from surgery and behavioural training as above, a microdialysis probe (2 mm CMA-7, cut off 6 kDa, CMA Microdialysis, Stockholm, Sweden) was inserted into the striatum through the guide cannula (the corresponding CMA-7 model). After insertion, probes were connected to a syringe pump and perfused at 1.2 μl min−1 with artificial cerebrospinal fluid (aCSF; Harvard Apparatus, Holliston, MA, USA). After a 30 min washout period and a subsequent 30 min pre-intake baseline sampling, dialysate samples were collected every 10 min and immediately manually injected into a HTEC-500 HPLC unit (Eicom, Japan). Analytes were then separated via an affinity column (PP-ODS, Eicom), and compounds subjected to redox reactions within an electro-chemical detection unit (amperometric DC mode, applied potential range from 0 to ∼2000 mV, 1 mV steps). Resulting chromatograms were analysed using the software EPC-300 (Eicom), and actual sample concentrations were computed based on peak areas obtained from a 0.5 pg μl−1 dopamine standard solution (Sigma) and expressed as percentage changes with respect to the mean dopamine concentration associated with the baseline (i.e. pre-behavioural task) sampling period. Locations of microdialysis probes were confirmed histologically.
Striatal dopamine receptor antagonism during intake tests
The D1/D2 dopamine receptor antagonist flupenthixol (Sigma) was infused bilaterally into the dorsal striatum (Murray et al. 2012) at the same site targeted by microdialysis probes at 15 μg 0.5 μl−1 per hemisphere. The drug was prepared fresh in aCSF (used as vehicle control) and infused 5 min prior to the 1 h oral glucose intake tests.
Sweetener intake coupled to intra-gastric infusions
Mice were trained to produce licks to a sucralose (2 mm)-containing spout to receive intra-gastric infusions of either sucralose (2 mm) or 1.4 m glucose. The exterior part of the gastric catheter was connected to a segment of MicroRenathane tubing secured to the tip of a 3 ml standard syringe containing the solutions to be infused and mounted on the syringe pump. The syringe pump was placed near a small hole made on the superior part of the sound attenuating box in such a way that mice could move freely inside the behavioural chambers. During the task, a detected sucralose lick triggered an intra-gastric infusion of the solution that lasted for 3 s at a rate of 0.6 ml min−1. However, licks detected while an infusion was taking place had no programmed consequences (i.e. did not result in additional infusions). Experimental tests lasted for 1 h. To train the animals in this task, once mice had recovered from surgery and been habituated to the behavioural chambers, they were habituated to lick for sucralose and obtain intra-gastric sucralose infusions. Training sessions lasted for 1 h and were performed daily under food (16 h) deprivation. Typically the animals had acquired stable responses after two or three sessions. Animals were considered trained to perform the experiments once they showed less than 20% between-session variability. Upon recovery and habituation, animals were assigned randomly to one of two experimental groups, such that licks for sucralose would result in either glucose or sucralose intra-gastric infusions. Animals were exposed to six daily 1-h sessions where they were allowed to consume ad libitum. Each of these sessions was preceded by i.p. injections of either vehicle or 2-DG on alternate days (i.e. Session 1: Vehicle; Session 2: 2-DG; Session 3: Vehicle; Session 4: 2-DG; Session 5: Vehicle; Session 6: 2-DG).
Statistical analyses
Data analyses were performed using SPSS (PASW Statistics Release 18.0.0) or Matlab (R14, MathWorks, Inc., Natick, MA, USA) and made use of linear mixed regression analyses as well as two- and one-way repeated measures analyses of variance (ANOVAs). Linear model analyses were performed to quantify the strength of the associations between relative dopamine efflux as measured by microdialysis and the numbers of licks produced. Data are reported as mean ± SEM.
Results
Ingestion of 0.8 m glucose, but not of 2 mm sucralose, markedly increases whole-body glucose oxidation rates
We started by confirming that consumption of our glucose stimulus, but not of the artificial sweetener sucralose, results in significant increases in glucose oxidation rates. Animals were placed in metabolic cages where, after 30 min of baseline measurements, ad libitum access to either the glucose or the sucralose solution was allowed. Indirect calorimetry measurements concomitant to sweet taste ingestion clearly shows that, while glucose ingestion rapidly and robustly leads to glucose-based nutrient utilization, sucralose ingestion did not produce relative changes in respiratory quotient values (Fig. 1A).
Figure 1. Glucoprivation reduces the intake levels of an artificial sweetener, but not of a glucose, solution.
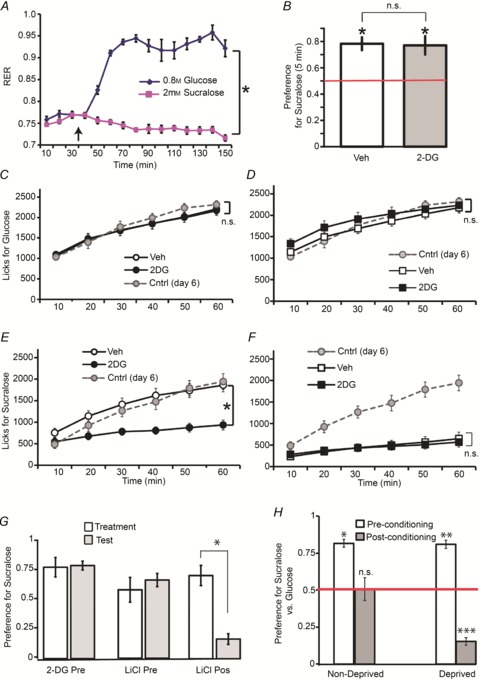
Data are depicted as mean ± SEM across animals in all figures. A, during indirect calorimetry sessions, 0.8 m glucose intake significantly shifted glucose oxidation rate from baseline values to physiologically maximal levels (respiratory exchange ratio (RER)∼1.0); however, 2 mm sucralose intake did not significantly shift glucose oxidation rate from baseline values. Within-subject stimulus effect on RER: *P < 0.0001 (n= 6). RER data were binned at 10 min intervals and either glucose or sucralose was introduced to the metabolic cages (vertical dark arrow) after 30 min of baseline sampling. B, in short-term (5 min) two-bottle preference tests, mice (n= 6) overwhelmingly preferred the taste of 2 mm sucralose over the taste of 0.8 m glucose (*independent t test against indifference ratio of 0.5 marked in red, *Bonferroni P < 0.004) irrespective of whether the preference session was preceded by glucoprivic (2-DG) injections (paired two-sample t test P > 0.6). C, during 1 h one-bottle 0.8 m glucose intake tests, mice (n= 15) did not modify their intake patterns after the 2-DG injection compared to after vehicle injection, as shown by the analyses of the timecourse lick data. Control mice (n= 7) injected daily with vehicle (shown in grey) did not differ from 2-DG treated animals. Note that data associated with the Vehicle condition are occluded by that of 2DG. D, similar effects were observed under the same conditions in recurrent mice (n= 15), i.e. mice that had been exposed to previous pairings between 2-DG injections and glucose consumption during the habituation phase. Note that intake patterns in recurrent animals treated with vehicle did not significantly differ from those observed in control mice treated daily with vehicle. E, however, during 1 h one-bottle 2 mm sucralose intake tests, a separate group of mice (n= 15) significantly reduced their intake patterns after the 2-DG injection compared to after vehicle injection (treatment × lick timecourse effect *P < 0.001). Intake was also significantly lower than in control mice (n= 7) treated daily with vehicle (shown in grey). F, in recurrent mice (n= 15), i.e. mice that had been exposed to previous pairings between 2-DG injections and sucralose consumption during the habituation phase (see Methods for details), no glucoprivation effect was observed. Intake was also significantly lower than in control mice treated daily with vehicle (shown in grey). G, we compared the ability of a malaise-inducing agent (lithium chloride = LiCl) to induce taste aversion in a classical conditioned taste aversion paradigm versus in the experimental design similar to the one described above. Only mice having received LiCl after the first 10 min sucralose versus water test decreased preferences on a subsequent similar test (‘LiCl Pos’, n= 8), whereas animals receiving either 2-DG (‘2-DG Pre’, n= 8) or LiCl (‘LiCl Pre’, n= 8) prior to the initial test (as in our paradigm) displayed unchanged preferences on a subsequent similar test (group × test day effect on sucralose preference *P= 0.002). H, groups of naïve food-deprived (n= 6) and non-deprived (n= 6) mice were exposed to an initial 5 min two-bottle sucralose versus glucose preference test as before, then to separate 1 h one-bottle sessions of ad libitum glucose or sucralose intake. Then after these 1 h one-bottle sessions the 5 min two-bottle sucralose versus glucose preference test was performed again. Mice strongly preferred sucralose versus glucose prior to the 1 h one-bottle sessions (post-hoc one-sample t tests again indifference ratio of 0.5 (red horizontal line) Bonferroni *P < 0.005; **P < 0.005). However, following the 1 h one-bottle sessions, while non-deprived mice showed indifference to the choice between sucralose and glucose, deprived mice displayed a robust preference for glucose, therefore completely reversing their initial preference for sucralose (Bonferroni ***P < 0.005). n.s., Non-statistically significant.
The taste of 2 mm sucralose is preferred over the taste of 0.8 m glucose during short-term two-bottle tests
Because we predicted greater decreases in sweetener intake compared to glucose intake upon disrupting glucose utilization with 2-DG, we were concerned with the possibility that such an effect could be confounded with changes in sweetener palatability. We have therefore intentionally chosen glucose and sucralose concentrations in such a way that the latter solution would be preferred over glucose solutions during short-term tests that minimize post-ingestive/metabolic influences on intake. In fact, 2 mm sucralose was overwhelmingly preferred over 0.8 m glucose during short-term 5 min two-bottle taste tests (independent t test against indifference ratio of 0.5, Bonferroni P < 0.004, Fig. 1B). Furthermore, these results remained unchanged after i.p. injections of 2-DG administered 10 min prior to the tests (paired two-sample t test P > 0.6, Fig. 1B). We therefore concluded that any 2-DG influences on sweetener versus sugar intake should not relate to changes in taste detection or preference.
Glucose deprivation produces enduring changes in sweetener, but not in glucose, intake
We then set out to test our hypothesis that glucose oxidation rates regulate the consumption levels of sweet tastants. We predicted that the intake levels of a sweet tastant would diminish if the tastant is paired to negative glucose oxidation (‘glucoprivic’) states. To test this prediction we presented mice with either glucose or sucralose solutions during 1 h one-bottle intake tests, where each session was preceded by an i.p. injection of either vehicle or 2-DG (see Methods for details).
Upon analysing the 1 h timecourse of the behavioural response, glucose intake levels were not influenced by 2-DG-induced glucoprivation (two-way repeated-measures ANOVA, drug treatment × lick timecourse effect F5,70= 0.29, P= 0.91, see Fig. 1C); overall intake under 2-DG was in fact similar to a control group of animals injected with vehicle in all sessions (group effect F1,20= 0.19, P= 0.66, glucose control group shown as grey line in Fig. 1C). In addition, in recurrent animals that had been exposed to previous pairings between 2-DG injections and glucose consumption during the habituation phase (see Methods for details), we similarly found no effects of 2-DG on glucose intake (F5,70= 1.3, P= 0.25), including when compared to the control group of animals (F1,20= 0.22, P= 0.64, see Fig. 1D).
However, we did observe robust inhibitory effects of glucoprivation on sucralose intake (>50% reduction compared to vehicle condition, F5,65= 20.8, P < 0.001, Fig. 1E). This is accounted for by the fact that sucralose intake does not promote glucose utilization (Fig. 1A). Consistently, sucralose intake under 2-DG was significantly lower than in a control group of animals injected with vehicle in all sessions (group effect F1,19= 12.2, P= 0.002, sucralose control group shown as grey line in Fig. 1E). Interestingly, we observed significantly lower levels of sucralose intake in recurrent animals (i.e. mice that had been exposed to previous pairings between 2-DG injections and sucralose consumption), irrespective of whether intake was preceded by vehicle or 2-DG injections (lick timecourse × treatment effect F5,65= 1.2, P= 0.27). In fact intake levels in recurrent animals after vehicle injections were approximately 50% of the intake levels of control naïve animals irrespective of day of testing (both group effects F1,19 > 24.0, P < 0.001, Fig. 1F). In other words, we have observed enduring yet specific glucoprivation-induced reductions in the motivation to ingest artificial sweeteners.
Glucoprivation-induced changes in sweetener intake are not due to conditioned taste aversion
To rule out the possibility that the inhibitory effect of 2-DG on artificial sweetener intake was due to conditioned aversion to sweetener taste, we compared the ability of a malaise-inducing agent (0.35 m lithium chloride) to induce taste aversion in a classical conditioned taste aversion paradigm versus in the experimental design similar to the one described above. One group of animals was treated with 2-DG 10 min prior to the beginning of 10 min two-bottle sucralose versus water preference tests. On the following day the same preference test was performed in the absence of drug treatment. Then, a second group of animals was exposed to the same protocol except that 0.35 m lithium chloride was used instead of 2-DG. Importantly, these two groups of non-naive animals had been exposed to sucralose versus water tests prior to the treatment day, in analogy to our main behavioural protocol. Finally, a third group of sweet-naïve animals was first exposed to the 10 min two-bottle sucralose versus water preference test and then treated with 0.35 m lithium chloride. A preference test was performed on the following day as above. We reasoned that if the suppression of sucralose intake induced by glucoprivation was due to conditioned aversion, a reduction in sucralose preference during the test day (compared to the preceding treatment day) would be observed in all groups. However, we observed a robust group × test day effect on sucralose preference (F2,15= 9.8, P= 0.002) due to the fact that only the third group (the ‘canonical’ conditioned aversion treatment) displayed significant reductions in sucralose preference on the test day compared to the preceding treatment day (see Fig. 1G). In fact, post-hoc comparisons show that the third group of animals produced significantly lower preference ratios on the test day compared to the other two groups (both Bonferroni P < 0.001). Importantly, sucralose preference in the non-naive groups (i.e. habituated animals treated with either 2-DG or lithium chloride prior to preference test in a preceding day) did not decrease during the test day (P= 0.21). We conclude that conditioned aversion is not induced in sweet-habituated animals that in addition received the drug treatment prior to exposure to sucralose on the test day, irrespective of whether the treatment is based on 2-DG or on a malaise-inducing agent.
Hunger shifts preferences away from palatable artificial sweeteners in favour of less palatable sugars
We also tested whether the artificial sweetener-specific effects shown above are solely due to the use of 2-DG, or alternatively are generalizable to physiological deprivations such as hunger. Groups of naïve food-deprived (n= 6) and non-deprived (n= 6) mice were exposed to an initial 5 min two-bottle sucralose versus glucose preference test as before, then to separate 1 h one bottle sessions of ad libitum glucose or sucralose intake. Then after these 1 h one bottle sessions the 5 min two-bottle sucralose versus glucose preference test was performed again (Fig. 1H). The overall test day × group effect for this experiment was F1,10= 12.2, P= 0.006. In fact, all mice as before strongly preferred sucralose versus glucose prior to the 1 h one-bottle sessions (post-hoc one-sample t tests against indifference ratio of 0.5 t5= 11.6, Bonferroni P < 0.005; t5= 11.0, P < 0.005). However, following the 1 h one-bottle sessions, while non-deprived mice showed indifference to the choice between sucralose and glucose (t5= 0.09, Bonferroni P= 0.92), deprived mice displayed a robust preference for glucose, therefore completely reversing their initial preference for sucralose (t5=−14.2, Bonferroni P < 0.005). Thus, deprived, but not non-deprived, mice completely switched their preferences in favour of glucose.
Extracellular levels of dopamine in mediodorsal striatum reflect the effects of oxidation rates on sweet taste intake
We next examined the effects of glucoprivation on dopamine efflux in mediodorsal striatum during the 1 h intake task described above. Dopamine levels increased significantly during glucose intake compared to pre-intake baseline levels, peaking at ∼40% above baseline at 40 min after vehicle injections (one-way repeated-measures ANOVA F6,84= 2.4, P= 0.03, Fig. 2A). However, dopamine levels did not differ from baseline levels under 2-DG treatment (F6,84= 2.0, P= 0.7); in fact a significant overall treatment effect was observed (F1,70= 30.6, P < 0.001). Interestingly, such an effect was not observed in recurrent animals ingesting glucose, for which dopamine levels remained close to baseline under both saline (F6,84= 0.53, P= 0.78; Fig. 2B) and 2-DG (F6,84= 0.83, P= 0.55) conditions, with no overall treatment effect being observed (F6,84= 2.3, P= 0.15).
Figure 2. Dopamine efflux in mediodorsal striatum reflects the effects of glucoprivation on sugar and artificial sweetener intake.
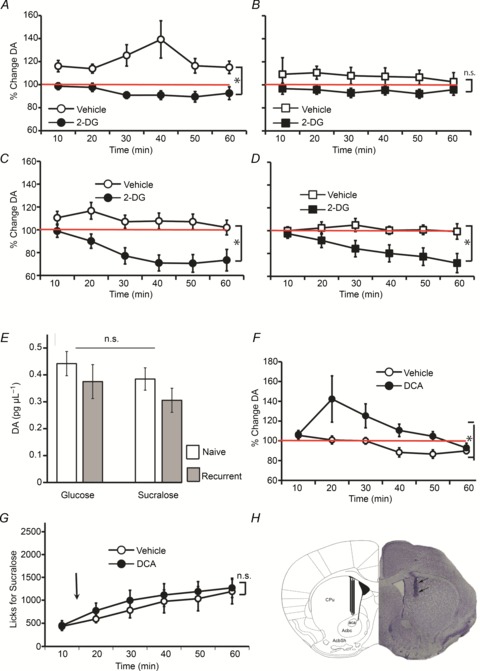
During the intake sessions depicted in Fig. 1C–F, microdialysis sampling from the mediodorsal striatum was performed concomitantly to intake monitoring. Baseline sampling was performed for ∼30 min prior to introduction of stimulus sippers to the cages. A, striatal dopamine levels were significantly higher than baseline levels during glucose intake after vehicle injections, whereas during glucoprivation levels were not significantly different from baseline (glucoprivation effect *P < 0.001). B, in recurrent mice, striatal dopamine levels were not significantly different from baseline levels during glucose intake irrespective of the glucoprivic treatment. C, striatal dopamine levels were not significantly different from baseline levels during sucralose intake after vehicle injections, whereas these levels were significantly lower than baseline during glucoprivation (glucoprivation effect *P= 0.002). D, similar effects were observed in recurrent animals ingesting sucralose (glucoprivation effect *P < 0.05). E, baseline dopamine levels did not differ across groups. F, mice (n= 6) ingesting sucralose following an injection of dichloroacetate (‘DCA’), which promotes glucose metabolism, displayed dopamine efflux significantly above baseline levels in contrast to following vehicle injections (treatment effect *P= 0.03). G, as expected, no significant behavioural modifications were observed in response to the dichloroacetate treatment. H, Histological analyses of brain tissue obtained from the animals that performed the 1 h behavioural + microdialysis tests reveal that dopamine sampling was restricted to the most medial aspect of the dorsal striatum region. A representative case is shown on the right side of the figure along with schematic representation of the final locations overlaid on the corresponding stereotaxic map. aca, Anterior commissure; AcbC, core region of the nucleus accumbens; AcbSh, shell region of the nucleus accumbens; CPu, caudate/putamen.
In contrast, in the sucralose group we observed no significant deviations from baseline following vehicle injections (F6,78= 1.8, P= 0.1; Fig. 2C). However, we observed significant decreases in dopamine levels during sucralose intake following 2-DG injections (dropping to 70% of baseline levels, F6,78= 8.7, P < 0.001). In fact a robust treatment effect was observed (F1,13= 13.90, P= 0.002). Similar patterns were observed in recurrent sucralose animals: we observed no significant deviations from baseline following vehicle injections (F6,78= 0.16, P= 0.98; Fig. 2D), but significant decreases in dopamine levels during sucralose intake following 2-DG injections (dropping to 70% of baseline levels, F6,78= 7.4, P < 0.001). In fact a treatment effect was detected (F1,13= 5.0, P < 0.05). These effects were not due to differences in dopamine absolute concentrations during baseline sampling (one-way ANOVA F3,54= 1.25, P= 0.3; Fig. 2E).
By performing a comparison across those sessions involving 2-DG injections, we observed a significant overall effect of condition (glucose vs. sucralose) on the dopamine timecourse response (sweetener condition × timecourse F15,270= 2.5, P= 0.001). This was due to the stronger suppressive effect of 2-DG on dopamine levels during sucralose compared to glucose intake, irrespective of whether groups were recurrent or naive. In fact, when recurrent animals in the glucose and sucralose conditions are compared directly, a robust effect is observed (two-way mixed-model ANOVA, sweetener condition × timecourse F5,135= 4.6, P= 0.001). Similar effects hold for naïve mice (F5,135= 2.5, P= 0.03).
We reasoned that if the effects of 2-DG on dopamine efflux were due to its inhibitory action on glucose utilization – rather than to unspecific aversive reactions – then combining sucralose intake with administration of compounds that enhance cellular glucose utilization should mimic the effects of glucose intake on dopamine efflux. Thus, an additional group of mice was exposed to the 1 h sucralose intake test following an intraperitoneal injection of dichloroacetate, a compound known to enhance glucose oxidation by stimulating the enzyme pyruvate dehydrogenase. Mice ingesting sucralose following the dichloroacetate injection displayed dopamine efflux significantly above baseline levels, such that a significant treatment effect was observed against the control vehicle condition (F1,5= 7.9, P= 0.03; Fig. 2F). As expected, no significant behavioural modifications were observed in response to the dichloroacetate treatment, which eliminates the possibility that its effects on dopamine efflux were due to aversive factors (Fig. 2G). Finally, histological analyses of probe placements revealed that microdialysis sampling was indeed restricted the mediodorsal aspect of the mouse striatum in all experiments (Fig. 2H).
In sum, our microdialysis results reveal that the ability of cellular glucose utilization to stimulate mediodorsal striatal dopamine efflux during sweetener ingestion in sweet-naïve predicts subsequent behavioural responses to the same sweetener during glucoprivation.
Relative changes in dopamine levels were not accounted for by oromotor patterns or other lick-related responses
One obvious concern regarding the above results relates to the possibility that dopamine levels simply reflect the number of lick responses produced by the animals during the behavioural/microdialysis sessions. Accordingly, to rule out the concern that the parallel between the behavioural and dopaminergic data was simply due to oromotor effects, we performed a series of linear association tests between the behavioural data shown in Fig. 1 and the concomitantly dopamine measures shown in Fig. 2 to assess the strength of the relationship between dopamine efflux relative to baseline and detected lick counts. We found no evidence for significant associations between licking behaviour and dopamine efflux in any of the four experimental groups tested, irrespective of treatment (Fig. 3).
Figure 3. Changes in mediodorsal striatum dopamine efflux during intake sessions were not accounted for by lick-related activity.
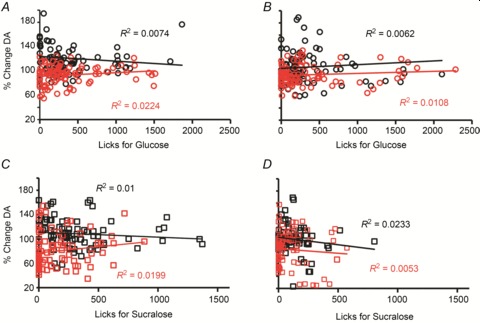
To rule out our own concern that the parallel between the behavioural and dopaminergic data was simply due to oromotor effects produced by licking rates on dopamine efflux, we performed a series of linear association tests to assess the strength of the relationship between dopamine efflux relative to baseline and detected lick counts. Overall, no significant associations between dopamine efflux and lick counts were observed during the glucose intake sessions in either naïve (A) or recurrent (B) mice. Similar findings hold for the sucralose sessions for both naïve (C) and recurrent (D) mice. Each panel displays the linear fit regression equation and corresponding R2 values separately for vehicle (black) and 2-DG (red) sessions (all P≥ 0.5). Lick counts and corresponding deviations of dopamine levels from baseline were binned with a 10 min resolution.
Dopamine antagonism during glucose intake in sweet-naïve animals mimics artificial sweetener ingestion during subsequent glucoprivation
If our contention that striatal dopamine release induced by glucose utilization controls ingestive responses during subsequent glucoprivation is correct, then it should be expected that blocking striatal dopamine signalling during the animal's first encounter with glucose should mimic the suppressing effects of subsequent glucoprivation on sweetener intake.
On the first testing day, the D1/D2 dopamine receptor antagonist flupenthixol or vehicle was infused bilaterally into the dorsal striatum at the same site targeted by the microdialysis probes 5 min prior to the 1 h oral glucose intake test. Mice were also treated i.p. with saline before the 1 h glucose test. On the second testing day, all animals were treated i.p. with 2-DG before the 1 h glucose test (no brain infusions were performed). As expected, glucose-naïve mice treated with striatal vehicle infusions on the first day of testing did not alter their intake patterns during glucoprivation (two-way repeated-measures ANOVA lick timecourse × testing day effect F5,25= 1.5, P= 0.21; see Fig. 4A). However, glucose-naïve mice treated with striatal flupenthixol infusions on the first day of testing significantly decreased their intake patterns during glucoprivation (F5,30= 7.5, P < 0.001; Fig. 4B). A control group of glucose-naïve mice treated with striatal flupenthixol infusions on the first day and i.p. with saline on both days did not alter their glucose intake patterns (F5,25= 0.84, P= 0.53; Fig. 4C). We therefore conclude that striatal dopamine receptor signalling during the first glucose intake test is the critical event determining insensitivity to devaluation during glucoprivation, which evidences a critical role for dopamine striatal signalling in controlling sweetener ingestion.
Figure 4. Dopamine antagonism during glucose intake in sweet-naïve animals mimics artificial sweetener ingestion during subsequent glucoprivation.
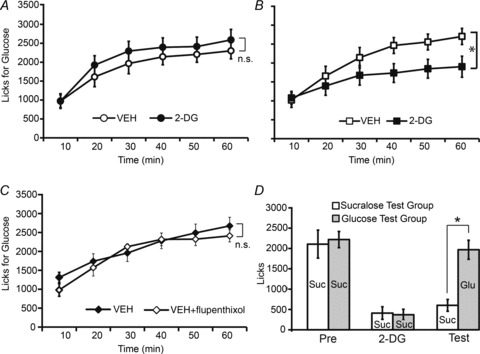
A, on a first testing day, vehicle was infused bilaterally into the dorsal striatum at the same site targeted by the microdialysis probes 5 min prior to the 1 h oral glucose intake test in sweet-naïve mice (n= 7). These mice were also treated i.p. with saline before the 1 h glucose test. On the second testing day, all animals were treated i.p. with 2-DG before the 1 h glucose test (no brain infusions were performed). Glucose-naïve mice treated with striatal vehicle infusions on the first day of testing did not alter their glucose intake patterns during glucoprivation. B, a different group of sweet-naïve mice (n= 6) was treated exactly as above except that the D1/D2 dopamine receptor antagonist flupenthixol was infused bilaterally into the dorsal striatum on the first day of testing. These mice significantly decreased their glucose intake patterns during glucoprivation (lick timecourse × testing day effect *P < 0.001). C, a control group of sweet-naïve mice (n= 6) treated with striatal flupenthixol infusions on the first day and i.p. with saline on both days did not alter their glucose intake patterns. D, two groups of mice (n= 6 and n= 5) were exposed to a 1 h sucralose test following an i.p. injection of saline on the first day of testing, and to a 1 h sucralose test following an i.p. injection of 2-DG on the second day of testing. Mice in both groups significantly decreased their sucralose intake during glucoprivation; however, on a third and final day of testing mice presented with glucose, but not those presented with sucralose, recovered their initial sweetener intake levels (group × testing day effect *P= 0.01). Text within bars indicates the sweet tastant consumed in each case (Suc, sucralose; Glu, glucose).
We also performed the converse experiment: we assessed the possibility that mice exposed to sucralose under glucoprivation would reinstate their initial intake levels on a subsequent test when challenged with glucose (i.e. under robust dopamine efflux in dorsal striatum). Two groups of animals were exposed on the first day of testing to a 1 h sucralose test following an i.p. injection of saline as before. The same mice were again presented on the second day with sucralose, but this time an i.p. injection of 2-DG was used. Finally, on the third testing day, one group was presented with a 1 h sucralose test and the other group with a 1 h glucose test. As expected, mice in both groups significantly decreased their sucralose intake during glucoprivation; however, and rather strikingly, on the third and final day of testing mice presented with glucose recovered their initial sweetener intake levels, with glucose-ingesting mice consuming significantly more than their sucralose-ingesting counterparts (mixed-model two-way ANOVA group × testing day effect F1,9= 10.1, P= 0.01; Fig. 4D). We conclude from this experiment that promoting dopamine efflux after a sweetener–glucoprivation pairing is sufficient to reinstate sweetener intake. This result also adds evidence against conditioned aversion effects associated with the glucoprivation-induced reductions in sucralose intake, as it rules out generalizations to other sweet tastants.
Glucoprivation effects on artificial sweetener intake were not due to orosensory factors
While our short-term tests (Fig. 1B) indicate that 2-DG injections are unlikely to modify the orosensory properties of artificial sweeteners, it is conceivable that the results presented above were due to slow-onset effects of glucoprivation on sucralose taste or to conditioned taste aversion-like mechanisms. To disambiguate this issue, we performed further experiments where all animals licked to sucralose-containing sippers; however, animals had also been implanted with gastric catheters such that detected licks for sucralose triggered either glucose or sucralose gut infusions. Animals assigned to either of these two experimental groups were exposed to six daily 1 h intake sessions, when they were allowed to consume ad libitum. To induce the effects of the recurrent glucoprivation treatment, each of these sessions was preceded by an i.p. injection of either vehicle or 2-DG on alternate days (i.e. Session 1: Vehicle; Session 2: 2-DG; Session 3: Vehicle; Session 4: 2-DG; Session 5: Vehicle; Session 6: 2-DG).
Similar to the results shown in Fig. 1C and D, intake levels remained stable when sucralose intake was linked to intra-gastric glucose infusions irrespective of type and recurrence of treatment. Specifically, mice licking sucralose and obtaining intra-gastric infusions of glucose sustained stable levels of intake throughout the six sessions, as shown by the analysis of the session-specific numbers of licks for sucralose (mixed-model two-way ANOVA, main effect of glucoprivic treatment F1,7= 0.03, P= 0.85; Session effect F2,14= 1.7, P= 0.2, Treatment × Session effect F2,14= 0.54, P= 0.59; Fig. 5A). Similar effects are observed when the number of sucralose-triggered intra-gastric (glucose) infusions are analysed (main effect of glucoprivic treatment F1,7= 1.0, P= 0.34; Session effect F2,14= 0.97, P= 0.4, Treatment × Session effect F2,14= 1.2, P= 0.32; Fig. 5B). However, and similarly to the results shown in Fig. 1E and F, we observed that a different group of mice licking sucralose and obtaining intra-gastric infusions of sucralose was associated with significant decreases in intake throughout the six sessions (main effect of glucoprivic treatment F1,7= 2.9, P= 0.13; Session effect F2,14= 12.0, P= 0.001; Treatment × Session effect F2,14= 0.24, P= 0.78; Fig. 5C). Similar effects were observed when the number of sucralose-triggered intra-gastric (sucralose) infusions were analysed (Main effect of glucoprivic treatment F[1,7]= 3.8, P= 0.09; Session effect F2,14= 28.4, P < 0.001, Treatment × Session effect F2,14= 1.0, P= 0.37; see Fig. 5D). Consistently, note that the absence of glucoprivic treatment (and session × treatment interaction) effects in Fig. 5C and D is accounted for by the fact that decreases in sucralose ingestion were elicited on the first glucoprivic session and intake remained low throughout the experiment irrespective of treatment.
Figure 5. Glucoprivation effects on artificial sweetener intake were not due to orosensory factors.
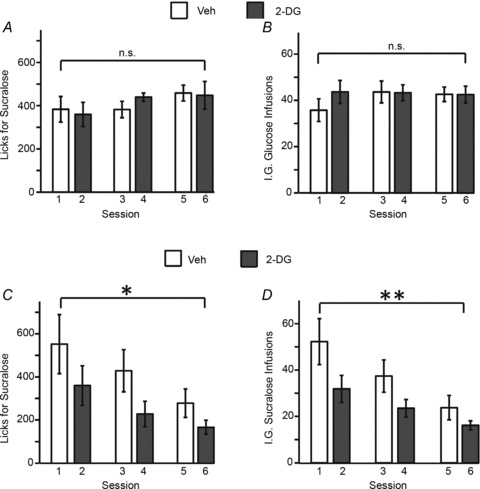
Mice licked to sucralose-containing sippers such that detected licks for sucralose triggered either glucose or sucralose intra-gastric infusions. To induce the effects of recurrent glucoprivation, each of the six daily sessions was preceded by an i.p.injection of either vehicle or 2-DG on alternate days. A, animals (n= 8) licking sucralose and obtaining intra-gastric infusions of glucose sustained stable levels of intake throughout the six sessions. B, similar effects are observed when the number of sucralose-triggered intra-gastric (glucose) infusions is analysed. C, however, for a different group of mice (n= 8), licking sucralose and obtaining intra-gastric infusions of sucralose was associated with significant decreases in intake throughout the six sessions (session effect *P= 0.001. D, similar effects are observed when the number of sucralose-triggered intra-gastric (sucralose) infusions are analysed (session effect **P < 0.001). Note that the absence of glucoprivic treatment (or session × treatment interaction) effects in C and D is accounted for by the fact that decreases in sucralose ingestion were elicited on the first glucoprivic session and intake remained low throughout the experiment irrespective of treatment (vehicle or 2-DG), pointing to enduring adaptive changes in motivation to consume artificial sweeteners that were triggered by the first sweetener–glucoprivation pairing.
At this point, it is important to consider the possibility that any decreases in sucralose intake (during oral-only sessions or when combined with intra-gastric sucralose infusions) observed on experimental day 2 (i.e. upon the first 2-DG injection) may not be due to glucoprivation per se, but simply to a lowering in the motivation to ingest the sweetener that may develop over the sessions. To rule out this potential confound, we collected intake data from three experimental sessions where animals (n= 8 in each group) licked sucralose to obtain intra-gastric infusions of either sucralose or glucose as above; however, sessions were such that behaviour was always preceded by vehicle injections. We found no effects of session day on sucralose intake levels under these conditions (mixed-model two-way ANOVA session effect F2,28= 0.27, P= 0.76; group effect F1,28= 0.003, P= 0.95; group × session interaction effect F2,28= 0.05, P= 0.95). We therefore conclude that the above session-related decreases in sucralose intake under the same conditions were accounted for only by the induction of glucoprivation.
Discussion
We have shown that the induction of glucoprivation in mice produces enduring decreases in artificial sweetener, but not sugar, intake. The glucoprivic effect on sweetener intake was not related to anti-metabolic interferences on sweet perception or sensory conditioned aversions to sweetener. From a neurobiological standpoint, our data reveal that glucose utilization rates control subsequent sweetener intake during glucoprivation by regulating mediodorsal striatal dopamine levels. More generally, our data thus suggest that mediodorsal striatal dopamine signalling controls the sensitivity of the animals to subsequent devaluations of sweet solutions by glucoprivation.
Our data are consistent with previous studies revealing the impact of glucoprivation on the nigrostriatal pathway. Thus, Parkisonian-like 6-hydroxydopamine-treated rats became akinetic when made glucoprivic, while dopamine receptor agonists were found to reverse these effects (Stricker et al. 1977; Snyder et al. 1985). Furthermore, dopamine-deficient mice display a blunted feeding response to 2-DG (Hnasko et al. 2004), although these authors report an inability to reverse this effect when dopamine signalling was restored in discrete caudate regions. Finally, our own previous results have shown that i.v. administration of 2-DG in non-behaving preparations robustly suppresses dopamine efflux in dorsal striatum, an effect that was reversed by infusions of glucose via the same route (Ren et al. 2010). The present study is, however, the first to probe dopamine efflux under glucoprivation (and stimulated glucose utilization) during the active intake of sweet solutions.
The fact that glucoprivation, a manipulation well known to alter feeding responses (Ritter et al. 1982; Benoit & Davidson, 1996; Hnasko et al. 2004; Darling & Ritter, 2009), impacts on dorsal striatum dopamine efflux is also consistent with a major role for this dopamine-targeted region in controlling goal-directed behaviours. In addition to normal dorsal striatum dopamine signalling being required for feeding (Sotak et al. 2005), integrity of dorsal striatal circuits is more generally necessary for the acquisition and expression of instrumental actions (whereas this does not hold for the nucleus accumbens of the ventral striatum, Yin et al. 2008). Furthermore, a functional dissociation exists within the dorsal striatum such that lesions to its medial part render animals insensitive to action–outcome devaluation or degradation, whereas lesions to its more lateral aspect impair the development of habits (Yin et al. 2004, 2005, 2006). Consistently, our data indicate that the amount of metabolism-induced increases in mediodorsal striatal dopamine levels produced during ingestion of a novel sweet tastant determines the extent to which animals will be sensitive to physiological degradation by glucoprivation, versus showing nutritional deficit-insensitive, habitual responses to the sweet tastant. At this point it is relevant nevertheless to stress that, while we have concentrated on dorsal striatal function for the reasons stated above, the ventral striatum also constitutes a critical regulatory circuit in motivated feeding (Mogenson et al. 1980; Berridge, 1996). Especially relevant for the present work, studies in rodents have implicated dopaminergic signalling in the nucleus accumbens of the ventral striatum in flavour-nutrient conditioning (Touzani et al. 2010). It is therefore conceivable that dissociations exist between ventral versus dorsal striatal functions in the control of energy-driven food reward: while the former may be critical for the formation of sensory cue–calorie associations, the latter may be primarily involved in the switch between goal-directed and habitual calorie intake.
Surprisingly, we have found no significant increases in glucose licking during glucoprivation, suggesting that mice may have reached a limiting, ceiling level of glucose intake that cannot be exceeded without inducing hypoglycaemia. On the other hand, however, we have observed that recurrent treatment with the glucoprivic agent 2-DG produces long-lasting adaptations related both to enduring decreases in artificial sweetener intake and to dopamine function (possibly both efflux and uptake) during intake of sweet solutions. From a behavioural perspective, these persistent decreases in sweetener intake induced by repeated glucoprivation are consistent with the concept that sweet taste may guide ingestion by acting as Pavlovian cues that signal ensuing metabolic consequences (Swithers & Davidson, 2008; Swithers et al. 2009). In fact, sucrose-paired cues motivate behaviour more potently than saccharin-paired cues concomitantly to evoking enhanced phasic dopamine bursts (McCutcheon et al. 2012). Further support for a role for glucose-derived metabolism in striatal-dependent behavioural processes is provided by recent studies showing that genetic enhancement of dopaminergic tone potentiates the incentive value of sugars while producing minimal impact on sweet-taste processing (Beeler et al. 2012). The current data therefore suggest that habitual artificial-sweetener consumers may relapse to sugar ingestion if artificial sweeteners are often consumed in a state of hunger or other physiological deprivation.
From a cellular standpoint, repeated administration of 2-DG is in fact known to induce molecular adaptations associated with intracellular neuronal energy sensing. Thus, repeated glucoprivation treatments reduce both subsequent hyperphagia and hyperglycaemia responses to 2DG along with decreased Fos immunoreactivity in both hypothalamus and adrenal medulla (Sanders & Ritter, 2000). Furthermore, repeated intracerebral 2-DG administration inhibited the action of the intracellular energy sensor 5′-adenosine monophosphate-activated protein kinase (AMPK), along with impaired counterregulatory hormonal responses (Alquier et al. 2007). Adaptations in AMPK activation in dopamine cells constitute one attractive hypothesis for the behavioural and neurochemical reworking observed in our preparation. It is also important to consider the possibility that glucoprivation action on dopamine efflux may result from rapid and/or long-lasting effects on dopamine transporter activity. In fact, recent studies point to insulin signalling (via PI3-K and Akt elements) as one major modulator of dopamine transporter activity (Garcia et al. 2005; Williams et al. 2007). It is thus plausible that the anti-glucose agent 2-DG may indirectly interfere on extracellular dopamine uptake by altering insulin signalling. Future studies must characterize the molecular mechanisms linking repeated cellular glucoprivation to adaptations in dopamine mechanics.
The considerations above raise the more general problem of which physiological pathways link glucose sensors to dopaminergic circuits. On the one hand, experiments employing flavour–nutrient conditioning paradigms suggest that small intestine nutrient stimulation may be required for animals to develop robust flavour preferences, whereas ileal or post-intestinal hepatic portal vein stimulation are not required (Ackroff et al. 2010). However, using a different preparation, a recent study suggests that intra-portal (and to some extent intra-jugular) infusions of glucose modulate both ingestive choice and phasic dopamine efflux (Oliveira-Maia et al. 2011). In addition to being consistent with our own previous findings (Ren et al. 2010), these results indicate that the site of glucose sensing relevant for dopaminergic efflux may be located within the CNS. In fact, Berthoud and Mogenson (1977) showed that 2-DG infused into the lateral ventricles of satiated rats elicited feeding. Furthermore, Granneman and Friedman (1983), using cerebral ventricular infusions of 2-DG, showed that prior disruption of cerebral metabolism is sufficient to elicit feeding in the absence of an adrenomedullary response. In this sense, it would be important to assess the dopaminergic effects produced by anti-metabolic agents such as 2,5-anhydro-d-mannitol, which unlike 2-DG is known to impact on feeding via peripheral pathways (Grill et al. 1995).
Regarding the above, two possibilities exist for how cerebral glucosensing may modulate dopaminergic function. On the one hand, non-dopaminergic brain circuits (via their axonal projections to the midbrain) may link neuronal nutrient sensing to dopamine efflux. In fact, hindbrain circuits containing catecholaminergic cell groups are known to detect glucose deficits (Ritter et al. 1981; Hudson & Ritter 2004; Watts & Donovan, 2009) and are required for expression of both the consummatory and the appetitive phases of glucoprivic feeding (Ritter et al. 2006). It is therefore of great interest to inquire whether midbrain-projecting hindbrain neurons reach dopaminergic areas, thereby mediating the suppressive effects produced by 2-DG on dopamine release. Another relevant possibility involves a role for nutrient-sensing hypothalamic neurons (Blouet & Schwartz, 2010) known to send efferent fibres into midbrain dopamine cells (Zheng et al. 2007). Specifically, AgRP neurons of the arcuate nucleus have been recently proposed to regulate dopaminergic function (Dietrich et al. 2012), and may therefore link cellular glucoprivation to dopamine efflux deficits.
An alternative hypothesis to the above states that dopaminergic cells of the midbrain may be under the direct influence of the intracellular availability of glucose, i.e. currently unidentified intracellular nutrient sensors may regulate the synthesis and release of neurotransmitters. In fact, intracellular nutrient sensing in dopamine neurons is suggested by the seminal discoveries that dopaminergic neurons of the substantia nigra robustly respond to locally applied glucose inflow (Levin, 2000) and that local glucose regulates substantia nigra GABA release via ATP-sensitive channels (During et al. 1995). These findings are consistent with exciting recent mouse studies revealing that K-ATP channels specifically expressed in dopaminergic substantia nigra neurons control bursting and novelty-induced exploration (Schiemann et al. 2012). As mentioned above, different intracellular nutrient sensors arise as relevant candidates, including AMPK (Horvath et al. 2009), which detects intracellular nutrient depletion via rises in AMP/ATP ratios (as in the case of the hindbrain, Li et al. 2011). Future investigations may thus contribute to establish glucose utilization as one new metabolic factor that acts to regulate dopaminergic neuronal function (Fulton et al. 2006; Hommel et al. 2006; Figlewicz et al. 2007).
Finally, it is important to stress that the notion that the reward value of sugars is sensed via detection of cellular energy utilization may be extended to several species including invertebrates. A series of fascinating recent studies independently report that flies not only survive by feeding on a tasteless metabolizable sugar (Dus et al. 2011), but also form odour-sugar memories only when sugar cues provide metabolic benefit (Burke & Waddell, 2011; Fujita & Tanimura, 2011; Wright, 2011). It is intriguing to note that in Drosophila, as in mammals, dopaminergic pathways play a role in regulating behavioural responses to rewarding stimuli (Burke et al. 2012). It is therefore tempting to speculate that glucose sensing takes place in dopaminergic neurons in non-mammal species.
We have shown that glucose metabolism regulates extracellular dopamine levels in dorsal striatum to control intake levels of sweet tastants. Future research must determine the identity of the cells mediating nutrient sensing actions on dopamine function.
Acknowledgments
None declared.
Glossary
- aCSF
artificial cerebrospinal fluid
- AMPK
5′-adenosine monophosphate-activated protein kinase
- 2-DG
2-deoxy-d-glucose
Additional information
Competing interests
None declared.
Author contributions
L.T. and X.R. performed the research, analysed and interpreted the data, and participated in writing the manuscript. W.H. and S.M. performed gastric and stereotaxic surgeries, and participated in performing, analysing and interpreting the data. J.F. performed histological analysis and participated in analysing the data. C.W.Y. participated in designing research, interpreting data and writing the manuscript. I.E.A. conceived the research, wrote the manuscript and participated in analysing and interpreting the data. All authors read and approved the submitted version of the manuscript.
Funding
This study was supported by NIH grant DC009997 to I.E.A.
References
- Ackroff K, Yiin YM, Sclafani A. Post-oral infusion sites that support glucose-conditioned flavor preferences in rats. Physiol Behav. 2010;99:402–411. doi: 10.1016/j.physbeh.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alquier T, Kawashima J, Tsuji Y, Kahn BB. Role of hypothalamic adenosine 5′-monophosphate-activated protein kinase in the impaired counterregulatory response induced by repetitive neuroglucopenia. Endocrinology. 2007;148:1367–1375. doi: 10.1210/en.2006-1039. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res. 2009;199:43–52. doi: 10.1016/j.bbr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Beeler JA, McCutcheon JE, Cao ZF, Murakami M, Alexander E, Roitman MF, Zhuang X. Taste uncoupled from nutrition fails to sustain the reinforcing properties of food. Eur J Neurosci. 2012;36:2533–2546. doi: 10.1111/j.1460-9568.2012.08167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit SC, Davidson TL. Interoceptive sensory signals produced by 24-hr food deprivation, pharmacological glucoprivation, and lipoprivation. Behav Neurosci. 1996;110:168–180. [PubMed] [Google Scholar]
- Berthoud HR, Mogenson GJ. Ingestive behavior after intracerebral and intracerebroventricular infusions of glucose and 2-deoxy-D-glucose. Am J Physiol. 1977;233:R127–133. doi: 10.1152/ajpregu.1977.233.3.R127. [DOI] [PubMed] [Google Scholar]
- Blouet C, Schwartz GJ. Hypothalamic nutrient sensing in the control of energy homeostasis. Behav Brain Res. 2010;209:1–12. doi: 10.1016/j.bbr.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- Burke CJ, Huetteroth W, Owald D, Perisse E, Krashes MJ, Das G, Gohl D, Silies M, Certel S, Waddell S. Layered reward signalling through octopamine and dopamine in Drosophila. Nature. 2012 doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke CJ, Waddell S. Remembering nutrient quality of sugar in Drosophila. Curr Biol. 2011;21:746–750. doi: 10.1016/j.cub.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling RA, Ritter S. 2-Deoxy-D-glucose, but not mercaptoacetate, increases food intake in decerebrate rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R382–386. doi: 10.1152/ajpregu.90827.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Ren X, Ferreira JG. Metabolic sensing in brain dopamine systems. Results Probl Cell Differ. 2010;52:69–86. doi: 10.1007/978-3-642-14426-4_7. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Bober J, Ferreira JG, Tellez LA, Mineur YS, Souza DO, Gao XB, Picciotto MR, Araujo I, Liu ZW, Horvath TL. AgRP neurons regulate development of dopamine neuronal plasticity and nonfood-associated behaviors. Nat Neurosci. 2012;15:1108–1110. doi: 10.1038/nn.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During MJ, Leone P, Davis KE, Kerr D, Sherwin RS. Glucose modulates rat substantia nigra GABA release in vivo via ATP-sensitive potassium channels. J Clin Invest. 1995;95:2403–2408. doi: 10.1172/JCI117935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dus M, Min S, Keene AC, Lee GY, Suh GS. Taste-independent detection of the caloric content of sugar in Drosophila. Proc Natl Acad Sci U S A. 2011;108:11644–11649. doi: 10.1073/pnas.1017096108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, MacDonald Naleid A, Sipols AJ. Modulation of food reward by adiposity signals. Physiol Behav. 2007;91:473–478. doi: 10.1016/j.physbeh.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Tanimura T. Drosophila evaluates and learns the nutritional value of sugars. Curr Biol. 2011;21:751–755. doi: 10.1016/j.cub.2011.03.058. [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Garcia BG, Wei Y, Morón JA, Lin RZ, Javitch JA, Galli A. Akt is essential for insulin modulation of amphetamine-induced human dopamine transporter cell-surface redistribution. Mol Pharmacol. 2005;68:102–109. doi: 10.1124/mol.104.009092. [DOI] [PubMed] [Google Scholar]
- Granneman J, Friedman MI. Feeding after recovery from 2-deoxyglucose injection: cerebral and peripheral factors. Am J Physiol. 1983;244:R383–388. doi: 10.1152/ajpregu.1983.244.3.R383. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Friedman MI, Norgren R, Scalera G, Seeley R. Parabrachial nucleus lesions impair feeding response elicited by 2,5-anhydro-D-mannitol. Am J Physiol. 1995;268:R676–682. doi: 10.1152/ajpregu.1995.268.3.R676. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Szczypka MS, Alaynick WA, During MJ, Palmiter RD. A role for dopamine in feeding responses produced by orexigenic agents. Brain Res. 2004;1023:309–318. doi: 10.1016/j.brainres.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Andrews ZB, Diano S. Fuel utilization by hypothalamic neurons: roles for ROS. Trends Endocrinol Metab. 2009;20:78–87. doi: 10.1016/j.tem.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Hudson B, Ritter S. Hindbrain catecholamine neurons mediate consummatory responses to glucoprivation. Physiol Behav. 2004;82:241–250. doi: 10.1016/j.physbeh.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Ann Rev Nutr. 1987;7:187–208. doi: 10.1146/annurev.nu.07.070187.001155. [DOI] [PubMed] [Google Scholar]
- Levin BE. Glucose-regulated dopamine release from substantia nigra neurons. Brain Res. 2000;874:158–164. doi: 10.1016/s0006-8993(00)02573-7. [DOI] [PubMed] [Google Scholar]
- Li AJ, Wang Q, Ritter S. Participation of hindbrain AMP-activated protein kinase in glucoprivic feeding. Diabetes. 2011;60:436–442. doi: 10.2337/db10-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Beeler JA, Roitman MF. Sucrose-predictive cues evoke greater phasic dopamine release than saccharin-predictive cues. Synapse. 2012;66:346–351. doi: 10.1002/syn.21519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobini S, Chambers LC, Yeomans MR. Effects of hunger state on flavour pleasantness conditioning at home: flavour-nutrient learning vs. flavour-flavour learning. Appetite. 2007;48:20–28. doi: 10.1016/j.appet.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Murray JE, Belin D, Everitt BJ. Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology. 2012;37:2456–2466. doi: 10.1038/npp.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Maia AJ, Roberts CD, Walker QD, Luo B, Kuhn C, Simon SA, Nicolelis MA. Intravascular food reward. PLoS One. 2011;6:e24992. doi: 10.1371/journal.pone.0024992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, De Araujo IE. Nutrient selection in the absence of taste receptor signaling. J Neurosci. 2010;30:8012–8023. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Wickens JR. Dopamine-dependent plasticity of corticostriatal synapses. Neural Netw. 2002;15:507–521. doi: 10.1016/s0893-6080(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213:451–452. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT, Li AJ. Hindbrain catecholamine neurons control multiple glucoregulatory responses. Physiol Behav. 2006;89:490–500. doi: 10.1016/j.physbeh.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Ritter S, Murnane JM, Ladenheim EE. Glucoprivic feeding is impaired by lateral or fourth ventricular alloxan injection. Am J Physiol. 1982;243:R312–317. doi: 10.1152/ajpregu.1982.243.3.R312. [DOI] [PubMed] [Google Scholar]
- Sanders NM, Ritter S. Repeated 2-deoxy-D-glucose-induced glucoprivation attenuates Fos expression and glucoregulatory responses during subsequent glucoprivation. Diabetes. 2000;49:1865–1874. doi: 10.2337/diabetes.49.11.1865. [DOI] [PubMed] [Google Scholar]
- Saris WH. Sugars, energy metabolism, and body weight control. Am J Clin Nutr. 2003;78:850S–857S. doi: 10.1093/ajcn/78.4.850S. [DOI] [PubMed] [Google Scholar]
- Schiemann J, Schlaudraff F, Klose V, Bingmer M, Seino S, Magill PJ, Zaghloul KA, Schneider G, Liss B, Roeper J. K-ATP channels in dopamine substantia nigra neurons control bursting and novelty-induced exploration. Nat Neurosci. 2012;15:1272–1280. doi: 10.1038/nn.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A. Post-ingestive positive controls of ingestive behavior. Appetite. 2001;36:79–83. doi: 10.1006/appe.2000.0370. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Touzani K, Bodnar RJ. Dopamine and learned food preferences. Physiol Behav. 2011;104:64–68. doi: 10.1016/j.physbeh.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AM, Stricker EM, Zigmond MJ. Stress-induced neurological impairments in an animal model of parkinsonism. Ann Neurol. 1985;18:544–551. doi: 10.1002/ana.410180506. [DOI] [PubMed] [Google Scholar]
- Sotak BN, Hnasko TS, Robinson S, Kremer EJ, Palmiter RD. Dysregulation of dopamine signaling in the dorsal striatum inhibits feeding. Brain Res. 2005;1061:88–96. doi: 10.1016/j.brainres.2005.08.053. [DOI] [PubMed] [Google Scholar]
- Stricker EM, Zimmerman MB, Friedman MI, Zigmond MJ. Caffeine restores feeding response to 2-deoxy-D-glucose in 6-hydroxydopamine-treated rats. Nature. 1977;267:174–175. doi: 10.1038/267174a0. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Baker CR, Davidson TL. General and persistent effects of high-intensity sweeteners on body weight gain and caloric compensation in rats. Behav Neurosci. 2009;123:772–780. doi: 10.1037/a0016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, Davidson TL. A role for sweet taste: calorie predictive relations in energy regulation by rats. Behav Neurosci. 2008;122:161–173. doi: 10.1037/0735-7044.122.1.161. [DOI] [PubMed] [Google Scholar]
- Szczypka MS, Mandel RJ, Snyder RO, Leff SE, Palmiter RD. Viral gene delivery selectively restores feeding and prevents lethality of dopamine-deficient mice. Neuron. 1999;22:167–178. doi: 10.1016/s0896-6273(00)80688-1. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. 6-Hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacology (Berl) 1986;90:390–397. doi: 10.1007/BF00179197. [DOI] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. Neuropharmacology of learned flavor preferences. Pharmacol Biochem Behav. 2010;97:55–62. doi: 10.1016/j.pbb.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG, Donovan CM. Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation. Front Neuroendrocrinol. 2009;31:32–43. doi: 10.1016/j.yfrne.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens JR, Begg AJ, Arbuthnott GW. Dopamine reverses the depression of rat corticostriatal synapses which normally follows high-frequency stimulation of cortex in vitro. Neuroscience. 1996;70:1–5. doi: 10.1016/0306-4522(95)00436-m. [DOI] [PubMed] [Google Scholar]
- Williams JM, Owens WA, Turner GH, Saunders C, Dipace C, Blakely RD, France CP, Gore JC, Daws LC, Avison MJ, Galli A. Hypoinsulinemia regulates amphetamine-induced reverse transport of dopamine. Plos Biol. 2007;5:2369–2378. doi: 10.1371/journal.pbio.0050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GA. Appetitive learning: memories need calories. Curr Biol. 2011;21:R301–302. doi: 10.1016/j.cub.2011.03.055. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Gould NJ, Leitch M, Mobini S. Effects of energy density and portion size on development of acquired flavour liking and learned satiety. Appetite. 2009;52:469–478. doi: 10.1016/j.appet.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behav Brain Res. 2006;166:189–196. doi: 10.1016/j.bbr.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur J Neurosci. 2008;28:1437–1448. doi: 10.1111/j.1460-9568.2008.06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


