Abstract
The aim of this study was to assess the extent of poly-pharmacy, occurrence, and associated factors for the occurrence of drug–drug interaction (DDI) and potential adverse drug reaction (ADR) in Gondar University Teaching Referral Hospital. Institutional-based retrospective cross-sectional study. This study was conducted on prescriptions of both in and out-patients for a period of 3 months at Gondar University Hospital. Both bivariate analysis and multivariate logistic regression were used to identify risk factors for the occurrence of DDI and possible ADRs. All the statistical calculations were performed using SPSS® software. A total of 12,334 prescriptions were dispensed during the study period of which, 2,180 prescriptions were containing two or more drugs per prescription. A total of 21,210 drugs were prescribed and the average number of drugs per prescription was 1.72. Occurrences of DDI of all categories (Major, Moderate, and Minor) were analyzed and DDI were detected in 711 (32.6%) prescriptions. Sex was not found to be a risk factor for the occurrence of DDI and ADR, while age and number of medications per prescription were found to be significant risk factors for the occurrence of DDI and ADR. The mean number of drugs per prescription was 1.72 and hence with regard to the WHO limit of drugs per prescription, Gondar hospital was able to maintain the limit and prescriptions containing multiple drugs supposed to be taken systemically. Numbers of drugs per prescription as well as older age were found to be predisposing factors for the occurrence of DDI and potential ADRs while sex was not a risk factor.
Keywords: Adverse drug reactions, drug-drug interactions, Ethiopia, gondar, poly-pharmacy, prescription
INTRODUCTION
Drug interactions may be defined as the combining of two or more drugs such that the potency or efficiency of one drug is significantly modified by the presence of another[1] and the concurrent use of multiple drugs for the treatment of different or the same conditions is known as poly-pharmacy.[2] Studies have shown that the risk of drug — drug interaction (DDI) and adverse drug reaction (ADR) has been found to increase markedly with the number of concomitant medications, which may also increase risks of impaired treatment efficacy and/or increased drug-related toxicity. In these studies, an increase of concomitant intake of drugs from 2 to 3 has been associated with an increase in the incidence of DDI to increase from 6% to 16%.[3]
Poly-pharmacy and adverse drug events continue to be difficult problems in modern healthcare despite continuing efforts to reduce their occurrence. Increased incidence of adverse drug events has been associated with a variety of healthcare environments, including the intensive care unit and various emergency settings (i.e., acute care surgery, emergency rooms, trauma care).[4] Patients treated with complex medical regimens in various combinations carry the potential for multiple DDI and ADRs. The frequent multiple co-morbidities in this patient population, including renal and liver dysfunction, poor nutritional status, and altered protein binding, amplify the risk of clinically significant drug interactions.[5]
Drug interactions are divided into three groups depending on the underlying mechanism of interaction: pharmacodynamic, pharmacokinetic, and pharmaceutical.[6] A pharmacodynamic interaction results from combining two drugs with similar mechanisms of action, which may behave in a synergistic, additive, or antagonistic manner.[7] A pharmacokinetic interaction occurs when a drug alters the pharmacokinetic processes such as absorption, distribution, metabolism (most often due to interaction with the cytochrome P450 hepatic enzymes), and/or excretion of another medication. A pharmaceutical interaction occurs when mixing chemically incompatible drugs outside the body, as for example, incompatibility of Phenobarbital with opioid analgesics when mixed in the same syringe, resulting in inactivation of one or both drugs.[8]
Majority of the literature on drug interactions published thus far describes pharmacokinetic interactions involving the CYP enzymes; in many cases with the use of drug probes.[9] DDIs can have three possible outcomes: increased therapeutic, adverse effects decreased therapeutic, or adverse effects or a unique response that does not occur with either agent alone.[10]
Clinicians, administrators, and policymakers have become interested in the system factors that contribute to ADRs and how prescribing practices can be improved. In a study done in United States outpatient setting, rates of ADRs due to DDI ranges from 2% to 50%.[11,12] Drug interactions are an example of inappropriate use of medication that may endanger the patient's health and this may be avoided by more careful prescribing. The clinical impact of the interaction may be most significant at the time of initial exposure. Awareness of potential drug interactions may permit development of clinical strategies to prevent their occurrence.[13]
The WHO considers the number of drugs per-prescription given to patients as one indicator and hence a measure of rational prescribing in a given health care setting. However, the extent of multiple prescribing, prevalence, and severity of DDI and problems associated with multiple drug prescribing is not well known in Ethiopia.
The present study was aimed to assess the extent of poly-pharmacy, prevalence of DDI, and associated ADR in Gondar University teaching Referral Hospital, North West Ethiopia. In addition, predisposing factors for the occurrence of DDI and ADR were studied using a multi-variate analysis and logistic regression.
MATERIALS AND METHODS
Study Design and Methodology
Institution-based retrospective cross-sectional study was conducted in Gondar University teaching referral hospital, North West Ethiopia. Gondar University hospital is a 450-bedded hospital and the only teaching referral hospital located in Amhara region, North-west Ethiopia. A retrospective analysis of the prescriptions was observed during the period of September 1 to November 30, 2012. The occurrence and severity of DDI and ADR were analyzed using Micromedex2® computer-based software. All the prescriptions dispensed to inpatients and out patients to all the patients from ≥6 months to 90 years age group from the central pharmacy of Gondar hospital were included in the study.
Demographic details and number of drugs prescribed were collected in a specially designed data collection proforma and the occurrence and severity of DDIs and ADRs were analyzed using Micromedex software. Associated factors for the occurrence of DDIs and ADRs were assessed using logical regression.
The study was approved by the Institutional Ethical review committee of Gondar University College of Medicine and Health sciences, University of Gondar, Ethiopia.
To ensure the quality data, one day training was provided to the research staff on research protocol and a pre-test was carried out to check the effectiveness of the data collection instrument and data was collected after standardizing the data collection instruments. All the statistical calculations were performed using SPSS® software.
Binary logistic regression was performed to analyze the association between predicting factors and the occurrence of DDI. Bivariate analysis of each predictor variables against the occurrence of DDI was also performed to identify the significant predictor variables that would qualify for the multivariate analysis. The major factors that were expected to determine the occurrence of DDI were first analyzed by considering the relationship of each predictor variable with the outcome variable.
RESULTS
Socio-Demographic Characteristics
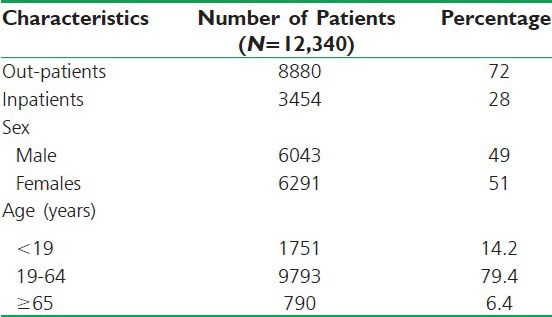
A total of 12,334 prescriptions were served during the study period both in the out-patient and inpatient pharmacy settings of Gondar University Hospital. Of these, 2180 prescriptions had more than one drug per prescription for systemic use. In 12,334 prescriptions, 6291 (51.0%) were females and 6043 (49.0%) were males. In these prescriptions, 1751 (14.2%) were children (<19 years) and 9793 (79.4%) were adults (19-64 years), while the remaining 740 (6.4%) of the patients were aged ≥65 years and the mean age of patients was found to be 36.4 ± 1.8 years. Moreover, 72% of the patients were out-patients and 28% of them are in-patients, respectively. The socio-demographic characteristics of patients are summarized in Table 1.
Table 1.
Socio-demographic characteristics of patients. (N=12,334)
Poly-Pharmacy, Occurrence of Drug-Drug Interaction, and Potential Adverse Drug Reactions
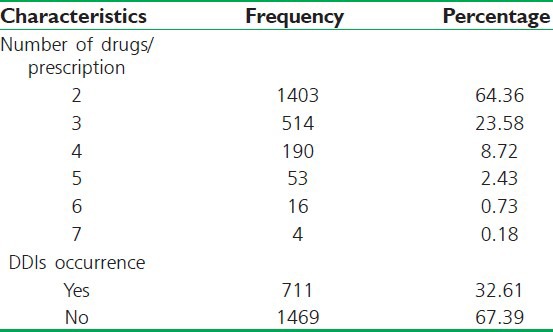
In our study, the maximum number of drugs per prescription was 7 while the minimum was 1 and the average number of drugs per prescription was found to be 1.72.
Occurrences of DDIs of all categories (Major, Moderate and Minor) were detected in 711 (32.61%) drugs of 2180 prescriptions containing two or more drugs while there were no any DDIs in 1469 (67.39%) of the prescriptions. A total of 1324 DDIs were detected in the 711 drugs of the 2180 prescriptions containing two or more drug regimens. The maximum numbers of 9 DDIs detected in 5 prescriptions and the minimum DDIs detected were 1 [Table 2].
Table 2.
Multiple prescribing, occurrence, and frequency of drug-drug interactions of all categories in prescriptions (N=2180)
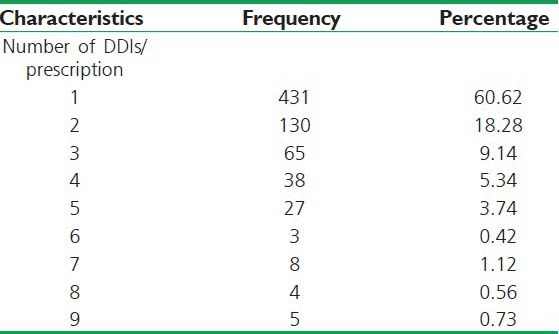
The number of DDIs of all categories detected in a prescription was also analyzed and the results are summarized in Table 3.
Table 3.
Total number of drug-drug interactions occurred per-prescription by all categories studied (N=711)
A total of 1324 DDIs are detected by using Micromedex software and were classified into three major categories as: Major, Moderate, and Minor. The total number of Major DDIs was found in 127 drugs, Moderate DDIs in 1020, and while minor DDIs accounts to 177. Moreover, a total of 11 contraindications (CIs) have been detected in 11 (0.83%) prescriptions and the majority of these contraindications (84.6%) were between amitriptyline and thioridazine prescribed together for the treatment of depression. This interaction causes a risk of CVS complications such as QT interval prolongation, torsades de pointes and cardiac arrest. Frequency and percent occurrence in each category of 1324 DDIs detected are summarized in Figure 1.
Figure 1.
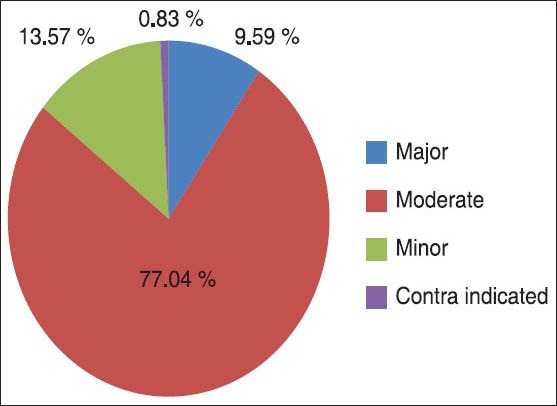
Pi-chart showing the percent occurrence of drug-drug interaction by severity among 1324 DDIs occurred on 711 prescriptions
Common Classes of Drugs Causing DDI and Common ADRs Detected
Over the study period, among the 2180 prescriptions reviewed, 1324 DDIs were detected and the possibility of the potential ADRs occurrence resulting from these DDIs was analyzed using the Micromedex software. Major and moderate DDIs detected were carefully reviewed for occurrence of possible ADR. The possible ADRs due to combination of the drugs were recognized by using Micromedex and were systematically categorized as following groups.
-
1)
ADR on CVS — risks of hypotension and hypovolemia, cardiac arrhythmias, torsade de pointes, postural hypotension, AV block, and possible digoxin toxicity (nausea, vomiting, cardiac arrhythmias).
-
2)
Metabolic disturbance — risk of hyperglycemia, hypoglycemia, hypertriglyceridemia, hyperkalemic lactic acidosis, lipodystrophy.
-
3)
Electrolyte disturbance — risks of hyperkalemia, hyponatremia, hypokalemia, hypercalcemia, hypomagnesaemia.
-
4)
ADRs on other organs such as hepatotoxicity, ocular toxicity, ADR on bones and others.
-
5)
CNS toxicity — increased risk of seizures, ataxia, nystagmus, diplopia, headache, seizures, and coma.
-
6)
Renal toxicity.
-
7)
ADRs on GIT — nausea, vomiting, constipation/diarrhea, and risk of gastrointestinal ulceration.
Summary of ADRs risks from Major and Moderate DDIs classified by the body system are summarized in Figure 2.
Figure 2.
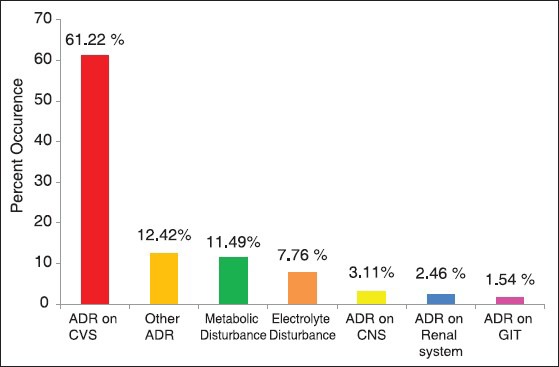
Possible ADRs occurrences from major and moderate DDIs using Micromedex software with prescriptions containing two or more drugs for systemic use (N= 1147)
Of the 1147 major to moderate ADRs detected by the Micromedex, therapeutic classes of drugs frequently associated with ADRs were cardiovascular diseases 485 (42.3%.), chemotherapeutic agents for systemic use 320 (27.9%), CNS diseases 242 (21.1%), non-steroidal anti-inflammatory drugs NSAIDs 52 (4.5%), and 48 (4.2%) ADRs constitutes to other classes of drugs. Summary of possible ADR occurrences from major and moderate DDIs classified by the body system are shown in Figure 2.
Factors Associated with Occurrence of DDIs
Bivariate analyses of each predictor variable against the occurrence of DDIs were performed to identify the significant predictor variable that would qualify for the multivariate analysis. The major factors that were expected to determine the occurrence of DDIs were first analyzed by considering the relationship of each predictor variable with the outcome variables. Two of the three explanatory variables considered in this study were found to be statistically significant for the occurrence of DDIs (P < 0.05). The results of these logisitic regression analysis revealed that age and number of drugs per prescription were found to be statistically significant in explaining the occurrence of DDIs. On the other hand, sex was not found to be a statistically significant factor for the occurrence of DDIs and possible ADRs. Table 4 provides the three predictors which were initially included in logistic regression model (multivariate analysis).
Table 4.
Logistic regression of the occurrence of DDIs with predictor variables on prescriptions studied in Gondar University Teaching Hospital
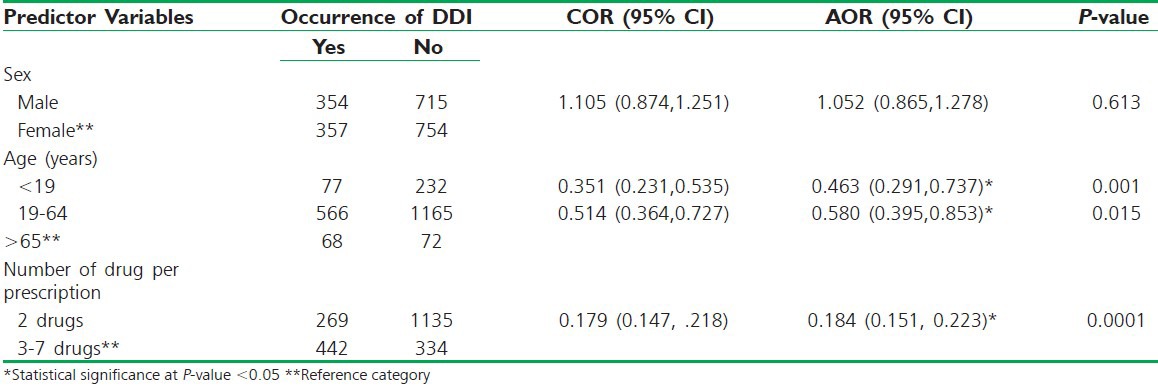
The adjusted logistic regression final model also showed that age and the number of drugs per prescription have a significant association with the occurrence of DDIs and possible ADRs. As shown in table 3, patients whose age is <19 years (46.3%) and those of age group 19 to 64 years (58%) were found to be less likely to encounter DDIs and possible ADRs than patients of ≥65 years (AOR = 0.463, 95% CI = 0.291-0.737 and AOR = 0.580 95% CI = 0.395-0.853), respectively.
Similarly, the occurrence of DDIs on prescriptions with two drugs (18.4%) are less likely than DDIs occurrence than those prescriptions containing three to seven drugs (AOR = 0.184 95% CI = 0.151-0.223). However, there was no significant difference in the occurrence of DDIs and possible ADRs with regard to sex.
DISCUSSION
Prescribing multiple drugs to patients at once (technically called poly-pharmacy) is not generally recommended as problems like dose missing, over dosing, DDIs, and ADRs may occur. Many medications have potential interactions with other drugs or substances when prescribed together. Studies have shown that increasing the number of drugs increases the risk of DDIs and ADRs exponentially.[14,15] WHO limits the average number of drugs per prescription to be within the range of 1.4-2.4 and the average number of drugs prescribed per prescription (encounter) in this study was found to be 1.72 showing the presence of acceptable prescribing practice based on WHO recommendations.[14]
Drug combinations with potential to interact are common in medical practice although their frequency in general medicine has been variable, depending on the patient population, study design, and the screening methods used to identify interactions. ADRs represent an important problem for hospital and primary care. Software that detects potential adverse drug interactions has been widely implemented in an effort to reduce the rate of ADEs. The use of electronic data resources and decision support to screen for DDIs is now the standard of practice for health care providers.[16] For this particular study, the software used for assessment was Micromedex®, a reliable software which gives evidence-based drug information about DDIs and potential ADRs categorizing into three classes (Major, Moderate, Minor) based on the mortality and morbidity probabilities on patients.
In this study, the occurrence of DDIs of all categories (Major, Moderate, and Minor) was analyzed and DDIs were detected in 711 (32.6%) of the 2180 prescriptions, comparable rate with other studies conducted elsewhere. In Brazil, a number of short-term studies reported on potential interactions among selected groups of drugs or patients and these reports suggest rates of DDIs occurred in 32% for pediatric and 22% for psychiatric patients.[17] In a study done in Canadian general medical wards, the rate of potential drug interactions has been approximately 60% which is higher rate compared to our study. Similar studies conducted in emergency departments found frequencies of potential drug interactions in the range of 16%-47%.[18] Another study done in Singapore showed that drug-related problems, which include ADRs, unnecessary drug therapy, inappropriate choice of drugs, and untreated conditions, have been shown to prevail in hospitalized patients, with a reported incidence rate as high as 25%.[19]
Common Classes of Drugs Causing DDI and Potential ADRs Detected
The consequences of DDIs can range from no untoward effects to drug-related morbidity and mortality. Although DDIs are considered preventable medication-related problems, studies have found that up to 11% of patients’ experience symptoms associated with DDIs and ADRs and are responsible for up to 2.8% of hospital admissions.[20] Research has also shown that DDIs are associated with increased health care costs. The economic burden of medication-related morbidity and mortality in the United States is estimated to be up to $130 billion annually.[21]
DDIs detected in this study were carefully reviewed for the occurrence of possible ADRs. The occurrences of ADRs were recognized by using Micromedex because of combined use of drugs were systematically categorized based on the risk on the body system where these ADRs could occur. Highly prevalent possible ADRs were encountered on the cardiovascular system such as risks of hypotension and hypovolemia, cardiac arrhythmias, QT prolongation which might progress into ventricular fibrillation, postural hypotension, AV block, and possible digoxin toxicity (nausea, vomiting, and cardiac arrhythmias) in 61.22% patients. But the study should be further supported by other follow-up studies for as it was done prospectively on patient medication records. Patient follow-up clinical studies conducted in other countries also reveal the same scenario though the prevalence seems to be lower. This might be due to the fact that all potential ADRs detected on the software may not occur on patients taking the medications. In a study performed at a Swedish university hospital, cardiovascular was the most affected system by ADRs compared to other organ systems constituting 36.3% of the total DRDs detected.[22]
Other possible ADRs detected in this study include metabolic disturbance such as risks of hyperglycemia, hypoglycemia, hypertriglyceridemia, hyperkalemic, lactic acidosis, and lipodystrophy. There were also risks of electrolyte disturbance like hyperkalemia, hyponatremia, hypokalemia, and hypercalcemia. Other risks of ADRs detected include hepatotoxicity, renal toxicity, and CNS toxicity such as increased risk of seizures, ataxia, nystagmus, diplopia, headache, seizures, and coma. ADRs on GIT such as nausea, vomiting, constipation, diarrhea, and risk of gastrointestinal ulceration were also noted during the analysis.
However, it should be noted that literature detailing drug interactions is often difficult to interpret, given the great disparities in how reactions are defined and the widely varying severity of responses among individuals. In addition, although many interactions between pharmacologic agents may be recognized using computer-based software and in theory, all of these interactions may not necessarily be clinically significant as occurrences of DDIs and ADRs can vary from individual to individual, based on disease condition, genetic makeup, and other factors. Hence, the above DDIs and ADRs detected are only potential hazards that can be used as precautionary measures for close supervision of patients or adjustment of treatment regimens.
Factors Associated with DDIs and ADRs
Indisputably, many factors can contribute to the high prevalence rate of DDIs and ADRs, but poly-pharmacy and older age have often been identified as imperative risk factors in this study. As the number of drugs prescribed at a time increases, there will be an increase in the probability of DDIs and ADRs, and the same is true with age. Over the last 20-30 years, problems related to aging, multi-morbidity, and poly-pharmacy have become a prominent issue in global healthcare.[23] A study done in the United States has shown that the incidence of DDIs and ADRs in the elderly has been reported to be two to three times the incidence in younger patients.[24] This increased risk for the elderly may be related to impaired organ reserve capacity, multiorgan dysfunction, altered pharmacokinetics, and pharmacodynamics. However, the most significant contributing factor for DDIs in this population was the frequency of poly-pharmacy.[25] Multiple medications may also result in unnecessary health expenditure, directly due to redundant drug sales and indirectly due to the increased hospitalization caused by drug-related problems. Drug-related problems are reported to cause between 10% and 20% of all emergency cases in hospitals and up to 20% of all admissions to hospitals of elderly patients.[26]
In line with the above studies, this study revealed that increasing the number of drugs in a prescription was found to be a significant factor for the occurrence of DDIs and potential ADRs. Similarly, aging was also found to be another significant risk factor for DDIs and ADRs, while sex was not found to be a predisposing factor. The adjusted logistic regression final model showed that age and number of drugs per prescription have a significant association with the occurrence of DDIs and possible ADRs.
As shown in table 4, patients whose age is less than 19 years and those in the age group of 19 to 64 years were found to be 46.3% and 58% less likely to encounter DDIs and possible ADRs than patients whose age was greater than or equal to 65 years (AOR = 0.463, 95% CI = 0.291-0.737 and AOR = 0.580 95% CI = 0.395-0.853), respectively. Similarly, the occurrence of DDI on prescriptions with two drugs was 18.4% less likely than the DDI occurrence on those prescriptions containing three to seven drugs (AOR = 0.184 95% CI = 0.151-0.223). However, there was no significant difference in the occurrence of DDIs and possible ADRs with regard to sex. Studies have demonstrated a correlation between increasing age, DDIs, and ADRs. This increased risk is related to the increased prevalence of chronic illnesses such as cardiovascular diseases, arthritis, and cancer.[27] Consequently, elderly are often treated with multiple medications causing high prevalence of DDIs and ADRs in these patient populations.
CONCLUSION
The results of this study showed that the mean number of drugs per prescription was 1.72 and hence with regard to the WHO limit of drugs per prescription, Gondar University Teaching Referral Hospital was able to maintain the limit. DDIs and potential ADRs were detected in 32.6% of those prescriptions containing two or more drugs for systemic use. Numbers of drugs per prescription as well as older age were found to be predisposing factors for the occurrence of DDIs and ADRs while sex was not a risk factor. Cardiovascular system was the most frequent site on which possible ADRs could be encountered as detected by using Micromedex software for this particular study.
RECOMMENDATIONS
Appropriate interventional strategies like educational, managerial, or regulatory should be made to practice evidence-based prescribing and close monitoring of patients taking drugs with potential DDIs and ADRs. Policy makers and stake holders should develop drug use policies and intervention measures such as implementation of computer-based software to be used in assisting clinical decision making. In addition, large-scale cross-sectional and follow-up studies should be conducted to assess extent of multiple prescribing, prevalence and severity of DDI, and associated problems nationwide.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Ellenhom J, Sternad A. Problems of drug interactions. J Am Pharm Assoc. 1996;6:65–8. doi: 10.1016/s0003-0465(15)31393-8. [DOI] [PubMed] [Google Scholar]
- 2.Viktil K, Blix H, Reikvam A. Poly-pharmacy as commonly defined is an indicator of limited value in the assessment of drug-related problems. Br J Clin Pharmacol. 2006;63:187–95. doi: 10.1111/j.1365-2125.2006.02744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frazier SC. Health outcomes and polypharmacy in elderly individuals: An integrated literature review. J Gerontol Nurs. 2005;31:4–11. doi: 10.3928/0098-9134-20050901-04. [DOI] [PubMed] [Google Scholar]
- 4.Stawicki P, Gerlach T. Polypharmacy and medication errors: Stop, Listen, Look, and Analyze. Scientist. 2009;3:6–9. [Google Scholar]
- 5.Nobili A, Garattini S, Mannucci PM. Multiple diseases and polypharmacy in the elderly: Challenges for the internist of the third millennium. J Co-morb. 2011;1:28–44. doi: 10.15256/joc.2011.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riechelmann RP, Zimmermann C, Chin SN, Wang L, O’Carroll A, Zarinehbaf S, et al. Potential Drug Interactions in Cancer Patients Receiving Supportive Care Exclusively. J Pain Sym Manage. 2008;35:535–43. doi: 10.1016/j.jpainsymman.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Jonker DM, Visser SA, van der Graaf PH, Voskuyl RA, Danhof M. Towards a mechanism-based analysis of pharmacodynamic drug – drug interactions in vivo. Pharmacol Ther. 2005;106:1–18. doi: 10.1016/j.pharmthera.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Reynolds KS, Zhao P, Huang SM. Drug interactions evaluation: An integrated part of risk assessment of therapeutics. Toxicol App Pharmacol. 2010;243:134–45. doi: 10.1016/j.taap.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Davies SJ, Eayrs S, Pratt P, Lennard MS. Potential for drug interactions involving cytochromes P450 2D6 and 3A4 on general adult psychiatric and functional elderly psychiatric wards. Br J Clin Pharmacol. 2004;57:464–72. doi: 10.1111/j.1365-2125.2003.02040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blower P, Wit R, Goodin S, Aapro M. Drug–drug interactions in oncology: Why are they important and can they be minimized? Cri Rev Oncoland Hematol. 2005;55:117–42. doi: 10.1016/j.critrevonc.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Bates W, Spell N, Cullen D, Burdick E, Laird N, Petersen A. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997;277:307–11. [PubMed] [Google Scholar]
- 12.Nolan L, O’Malley K. Prescribing for the elderly. Part 1: Sensitivity of the elderly to adverse drug reactions. J Am Geriatr Soc. 1988;36:142–9. doi: 10.1111/j.1532-5415.1988.tb01785.x. [DOI] [PubMed] [Google Scholar]
- 13.Heininger-Rothbucher D, Bischinger S, Ulmer H, Pechlaner C, Speer G, Wiedermann CJ. Incidence and risk of potential adverse drug interactions in the emergency room. Resuscitation. 2001;49:283–8. doi: 10.1016/s0300-9572(00)00363-4. [DOI] [PubMed] [Google Scholar]
- 14.Wochenschr K. Poly-pharmacy, inappropriate prescribing and adverse drug reactions in Austria. Mid Euro J Med. 2008;120:713–4. doi: 10.1007/s00508-008-1106-2. [DOI] [PubMed] [Google Scholar]
- 15.Cadieux RJ. Drug interactions in the elderly. How multiple drug use increases risk exponentially? Postgrad Med. 1989;86:179–86. doi: 10.1080/00325481.1989.11704506. [DOI] [PubMed] [Google Scholar]
- 16.Wong K, Yu S, Holbrook A. A systematic review of medication safety outcomes related to drug interaction software. J Pop Ther Clin Pharmacol. 2010;17:243–55. [PubMed] [Google Scholar]
- 17.Aparasu R, Baer R, Aparasu A. Clinically important potential drug-drug interactions in outpatient settings. Res Soc Admin Pharm. 2007;3:426–37. doi: 10.1016/j.sapharm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Riechelmann R, Zimmermann C, Chin S, Wang L, Zarinehbaf S, Krzyzanowska M. Potential Drug Interactions in Cancer Patients Receiving Supportive Care Exclusively. J Pain Symptom Manage. 2008;35:535–43. doi: 10.1016/j.jpainsymman.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Stewart B, Cooper W. Poly-pharmacy in the aged. Practical solutions. Drugs Aging. 1994;4:449–61. doi: 10.2165/00002512-199404060-00002. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton RA, Briceland LL, Andritz MH. Frequency of hospitalization after exposure to known drug-drug interactions in a Medicaid population. Pharmacotherapy. 1998;18:1112–20. [PubMed] [Google Scholar]
- 21.Aparasu R, Baer R, Aparasu A. Clinically important potential drug-drug interactions in outpatient settings. Res Soc Admin Pharm. 2007;3:426–37. doi: 10.1016/j.sapharm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Mjörndal T, Boman MD, Hägg S, Bäckström M, Wiholm BE, Wahlin A, et al. Adverse drug reactions as a cause of admissions to a department of internal medicine. Pharmacoepidemiol Drug Saf. 2002;11:65–72. doi: 10.1002/pds.667. [DOI] [PubMed] [Google Scholar]
- 23.Nobili A, Garattini S, Mannucci M. Multiple diseases and polypharmacy in the elderly: Challenges for the internist of the third millennium. Journal of Comorbidity. 2011;1:28–44. doi: 10.15256/joc.2011.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nolan L, O’Malley K. Prescribing for the elderly. Part 1: Sensitivity of the elderly to adverse drug reactions. J Am Geriatr Soc. 1988;36:142–49. doi: 10.1111/j.1532-5415.1988.tb01785.x. [DOI] [PubMed] [Google Scholar]
- 25.Gaeta T, Fiorini M, Ender K, Bove J, Diaz J. Potential drug-drug interactions in elderly patients presenting with syncope. J Emerg Med. 2002;2:159–62. doi: 10.1016/s0736-4679(01)00471-1. [DOI] [PubMed] [Google Scholar]
- 26.Mjorndal T, Boman M, Hagg S, Backstrom M, Wiholm B, Wahlin A, Dahlqvist R. Adverse drug reactions as a cause for admissions to a department of internal medicine. Pharmacoepidemiol Drug Saf. 2002;11:65–72. doi: 10.1002/pds.667. [DOI] [PubMed] [Google Scholar]
- 27.Wang L. Epidemiology and prevention of adverse drug reaction in the elderly. J Ger Cardio. 2005;4:248–53. [Google Scholar]


