Figure 2.
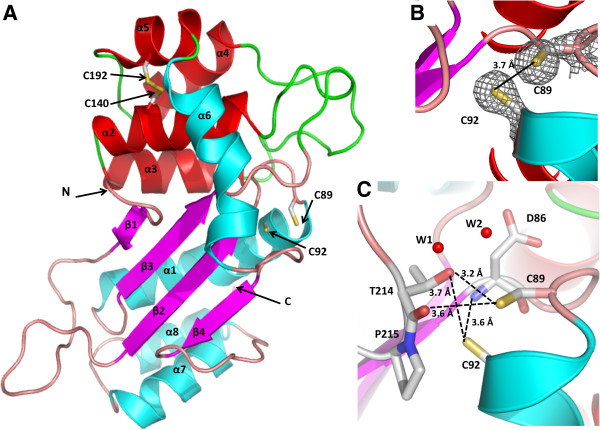
Crystal structure of Mt-DsbA reveals a DsbA-like fold. A. Cartoon representation of Mt-DsbA reveals a two-domain structure comprising a TRX domain (colored in pink for β-strands, cyan for α-helices and salmon for loops) and an inserted α-helical domain (colored in red for α-helices and green for loops). The overall fold is reminiscent of DsbA-like homologs. The CXXC motif cysteines, Cys89 and Cys92, are reduced, while the α-helical domain cysteines, Cys140 and Cys192, form a disulfide bond. B.2Fo-Fc electron density mesh (grey) of the active site CXXC cysteine residues contoured at 1σ. C. The reduced CXXC form of Mt-DsbA is stabilized by hydrogen bonds (designated with black dashed lines) to Thr214 of the cis-Pro loop.