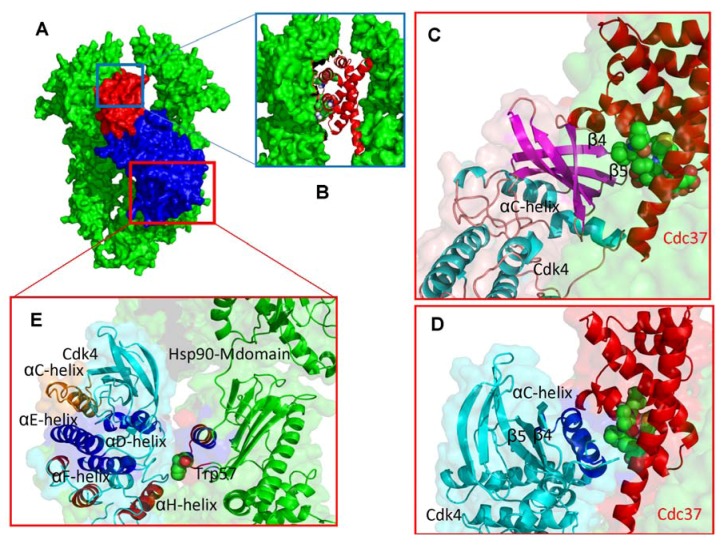
Figure 7.
Structural Mapping of the Intermolecular Interfaces in the Hsp90-Cdc37-Cdk4 Docked Complexes (A) Structural overview of the low-energy docked complexes. The Hsp90 dimer is shown in green, Cdc37 is colored in red and Cdk4 is displayed in blue. (B) A close-up of the intermolecular interface between M-domain of Cdc37 (ribbon/sticks representation, colored in red) and Hsp90-NTD (surface representation, colored in green). (C) Structural details of the Cdk4-Cdc37 interface from the first low energy binding mode. The Cdc37 motif 190–LVIWCI-195 interfaces with the β4 and β5 strands of the N-terminal kinase lobe. Cdc37 is shown in red ribbons and the interacting Cdc37 helix is shown in spheres with atom-based coloring scheme. The interacting elements of Cdk4 are in blue ribbons, the rest of Cdk4 in cyan ribbons. (D) The Cdk4-Cdc37 interface from alternative low energy binding mode. The αC-helix region of Cdk4 interacts with the Cdc37 recognition motif. (E) A close-up of the interface between the C-terminal kinase lobe and the middle domain of Hsp90.