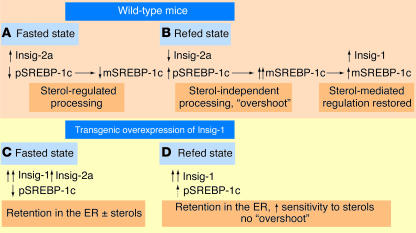
Figure 1.
Integration of metabolic signals at the ER membrane. (A) During fasting, expression of the SREBP-1c precursor (pSREBP-1c) is reduced. Insig-2a is expressed and binds to SCAP, causing retention of a very low amount of pSREBP-1c in the ER in the presence of sterols. (B) Upon refeeding, insulin increases the expression of pSREBP-1c and decreases the expression of Insig-2a. The low abundance of both Insig-1 and Insig-2a leads to greatly increased processing of pSREBP-1c to mature SREBP-1c (mSREBP-1c), leading to a lipogenesis rate that is more than tenfold higher than that of the basal state, which represents an “overshoot.” The increased level of mSREBP-1c promotes expression of Insig-1. At a critical stoichiometry relative to SCAP, Insig-1 restores sterol-mediated regulation of pSREBP-1c processing. The lipogenesis rate then returns to the original basal level. (C) With transgenic overexpression of Insig-1, the cycling of lipogenesis between the fed and fasted states is dampened. The high ratio of Insig-1 to SCAP causes retention of pSREBP-1c in the ER. Fasted mice have a modest additional drop in lipogenesis due to the reduction of pSREBP-1c expression and increased Insig-2a levels. (D) With refeeding, the transgenic mice express extremely low levels of pSREBP-1c due to their inability to produce enough mSREBP-1c to drive the transcription of the pSREBP-1c gene. The persistently elevated level of Insig-1 abolishes the dramatic “overshoot” in lipogenesis seen in the wild-type refed mice but still mediates an enhanced sensitivity of pSREBP-1c processing to sterols.