Abstract
This unthrifty heifer calf was thin, weak and had a dull haircoat. Urinalysis and blood work revealed glycosuria, ketonuria, hypoproteinemia, and hyperglycemia. Euthanasia and necropsy were performed, revealing multifocal intersitial lymphocytic infiltration, an absence of islet cells, and a positive stain for bovine viral diarrhea virus in the pancreas.
A January born Charolais heifer calf was presented to the Leduc Veterinary Hospital on June 11, 2002, with a history of progressive weight loss and general unthriftiness. Initially, she was a large, healthy calf that had been selected as a show animal. In a period of 3 wk to 1 mo, she had lost weight and become depressed. The owner noted that she seemed to urinate frequently. The calf was still on the cow; the owner had seen her nursing but did not think that there was an increased frequency in suckling. In this herd, the heifers were vaccinated twice in the fall against bovine rhinotracheitis virus, bovine viral diarrhea virus, parainfluenza-3 virus, respiratory syncytial virus, and Haemophilus somnus (Triangle 4; Ayerst Laboratories, Guelph, Ontario), and heifers and cows received booster vaccinations in the spring. This calf was born to a heifer. No vaccines had been administered to the calf.
On physical examination, the calf was thin, slightly weak, and had a dull hair coat. She was slightly depressed. Her temperature (38.5°C) and respiratory rate (36 breaths/min) were within normal limits, but her heart rate was slightly elevated (120 beats/min; normal, 100 to 120 beats/min). The calf was approximately 6% dehydrated (based on eye position and skin tent) and her manure was pasty. No other abnormal findings were noted on physical examination. Results of a urinalysis were within normal limits, except for ketones (16 mmol/L) and glucose (56 mmol/L). The only abnormalities noted on a complete blood cell (CBC) count were an elevated packed cell volume (0.47 L/L; normal, 0.26 to 0.44 L/L), a low total protein (54 g/L; normal, 70 to 85 g/L), and a slightly increased segmented neutrophil count (5.0 × 109/L; normal, 0.6 to 4.0 × 109/L).
On biochemical analysis, the glucose was elevated at (10.30 mmol/L; normal 3.11 to 4.89 mmol/L). The only other abnormality was a slightly low phosphorus level (61 g/L, normal 62 to 80 g/L).
At this time, a tentative diagnosis of diabetes mellitus was made and the owners were contacted. A decision was made to euthanize the animal and perform a necropsy.
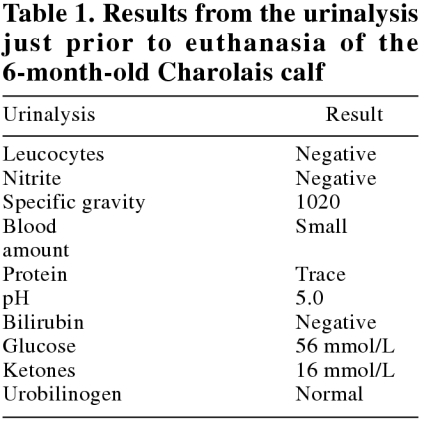
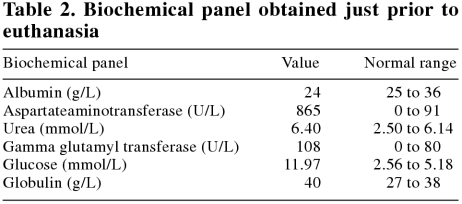
On June 18, 2002, the calf was brought to the clinic to be euthanized. She was still thin, and slightly weak. Her abdomen appeared quite empty. Her temperature, pulse, and respiration were 38.9°C, 60 beats/min, and 20 breaths/min, respectively. No other abnormalities were noted on physical examination. Another urinalysis (Table 1) and biochemical panel (Table 2) were carried out. The glucose and ketones were still very high, which is consistent with diabetes mellitus.
Table 1.
Table 2.
On necropsy, the calf's general body condition was poor. The liver was pale and friable. The pancreas appeared slightly smaller than normal. The renal cortices of both kidneys were pale. No other abnormalities were noted. Samples of pancreas, liver, kidney, spleen, duodenum, jejunum, spiral colon, mesenteric and bronchial lymph nodes, pituitary, bladder, heart, and lung were all submitted in buffered 10% formaldehyde for histopathological examination to the Central Veterinary Pathology Laboratory in Edmonton.
There was moderate multifocal interstitial infiltration by lymphocytes, a lesser number of plasma cells, and a few mixed leukocytes in the pancreas. No islets were evident. There was extensive hepatocellular cytoplasmic vacuolation, especially central lobularly in the liver. The kidney had moderate cytoplasmic vacuolation of tubular epithelial cells. No abnormalities were found in any of the other samples submitted. The final diagnosis was lymphocytic pancreatitis, which was consistent with immunemediated pancreatitis, and a secondary fatty liver and fatty change in the kidneys.
The pancreatic sample was later stained to check for bovine viral diarrrhea virus (BVDV) and the result was positive. A review of the ranch's vaccination program revealed that the heifers were not being vaccinated against BVDV infection until after they were bred. A recommendation was made to change the vaccination program to ensure that animals were vaccinated prior to breeding.
Diabetes mellitus has been reported in cattle, pigs, sheep, horses, and bison; it is relatively uncommon in cattle. There are several possible concurrent predisposing diseases or risk factors that are discussed in the literature, such as fatty liver, fat cow syndrome, parturition and chronic insulitis, as well as viral diseases, especially bovine virus diarrhea (1,2,3,4,5). Cases of diabetes mellitus have been reported in various breeds of cattle including Aberdeen Angus 3 Jersey (6), Hereford (1), Holstein-Friesian (1,7,8), brangus (2), Japanese black, and Japanese brown (3,4,9,10). Several studies have performed glucose tolerance tests and measured fructosamine levels to show that the hyperglycemia, glycosuria, and ketonuria have been due to a decrease in insulin production by the pancreas. The glucose disappearance rate and half time were markedly longer in cattle with diabetes than in normal control cattle. Fructosamine levels were also elevated in the diseased animals (3,9,11). Other causes of hyperglycemia that have been reported are enterotoxemia, parturient paresis, foot and mouth disease, botulism, septicemia, polioencephalomalacia, and rabies (12).
Diabetes mellitus in cattle is often immunemediated and is similar to juvenile onset diabetes mellitus in humans. In humans, there is often an underlying viral infection that causes an autoimmune reaction to the b cells of the pancreas (3). A genetic background for diabetes in humans has also been suspected, but the establishment of such a link in cattle has not been reported (4). Cases of diabetes have been reported in cattle infected with BVDV (3,4,5) and foot and mouth disease (13). No correlation has been made between the serotype of BVDV and the occurrence of diabetes mellitus (4).
Viral infection may cause diabetes mellitus by 2 different mechanisms: 1) the virus directly destroys b cells in the pancreas, or 2) an immune response against the virus infection may induce an autoimmune response in the host (4). The lack of insulin in animals with diabetes mellitus results in elevated glucose levels in the blood and urine. Due to the abnormal glucose metabolism and storage, there is mobilization of lipids from peripheral adipose tissue. Fatty acid synthesis in the liver is impaired in the diabetic animal and there is a buildup of ketone bodies, which eventually leads to acid-base balance impairment, ketoacidosis, and dehydration, resulting in collapse, coma, and death (2).
There are several different presentations of diabetes mellitus in cattle (1,5,8), with the most common being weight loss, poor hair coat, glucosuria, ketonuria and hyperglycemia. A glucose tolerance test can be performed and fructosamine levels can be examined to confirm the diagnosis. Treatment may be attempted, but it is probably not economically or practically viable. It is likely that most cases of diabetes mellitus are the result of a viral infection, often BVDV.
Footnotes
Acknowledgments
The author thanks Leduc Veterinary Hospital, particularly Dr. Steve Radostits and Dr. Wayne Sereda, for their cooperation with this case report. CVJ
Dr. Clark will receive 50 free reprints of her article, courtesy of The Canadian Veterinary Journal.
Address all correspondence and reprint requests to Dr. Clark.
Dr. Clark's current address is PO Box 447, Dawson Creek, British Columbia V1G 4H3
References
- 1.Gould AC. Diabetes mellitus in cattle. Vet Rec 1981;109:539. [DOI] [PubMed]
- 2.Kitchen D, Roussel A. Type-1 diabetes mellitus in a bull. J Am Vet Med Assoc 1990;197:761–763. [PubMed]
- 3.Taniyama H, Hirayama K, Kagawa Y, et al. Immunohistochemical demonstration of bovine viral diarrhoea virus antigen in the pancreatic islet cells of cattle with insulin-dependent diabetes mellitus. J Comp Pathol 1999;121:149–157. [DOI] [PubMed]
- 4.Tajima M, Yuasa M, Kawanabe M, Taniyama H, Yamato O, Maede Y. Possible causes of diabetes mellitus in cattle infected with bovine viral diarrhoea virus. J Vet Med 1999;46:207–215. [DOI] [PubMed]
- 5.Tajima M, Yazawa T, Hagiwara K, Kurosawa T, Takahashi K. Diabetes mellitus in cattle infected with bovine viral diarrhoea mucosal disease virus. J Vet Med Assoc 1992;39:616–620. [DOI] [PubMed]
- 6.Kaneko JJ, Rhode EA. Diabetes mellitus in a cow. J Am Vet Med Assoc 1964;144:367–373. [PubMed]
- 7.Wallace CE, Kociba GJ. Diabetes insipidus in a cow. J Am Vet Med Assoc 1979;175:809–811. [PubMed]
- 8.Mostaghni K, Ivoghli B. Diabetes mellitus in the bovine. Cornell Vet 1977;67:24–28. [PubMed]
- 9.Taniyama H, Hirayama K, Kagawa Y, et al. Histopathological and immunohistochemical analysis of the endocrine and exocrine pancreas in twelve cattle with insulin-dependent diabetes mellitus (IDDM). J Vet Med Sci 1999;61:803–810. [DOI] [PubMed]
- 10.Taniyama H, Ushiki T, Tajima M, et al. Spontaneous diabetes mellitus associated with persistent bovine viral diarrhea (BVD) virus infection in young cattle. Vet Pathol 1995;32:221–229. [DOI] [PubMed]
- 11.Hasegawa T, Uchida K, Yanase J, et al. A case of diabetes mellitus in Japanese black cattle. J Vet Med Sci 1999;61:965–966. [DOI] [PubMed]
- 12.Whitlock RH, Tasker JB. Hyperglycemia in ruminants. Bull Am Soc Vet Clin Pathol 1972;1:5–9.
- 13.Barbni E, Manocchio I, Asdrubali G. Observations on diabetes mellitus associated with experimental foot and mouth disease in cattle. Vet Ital 1966;17:339–368.