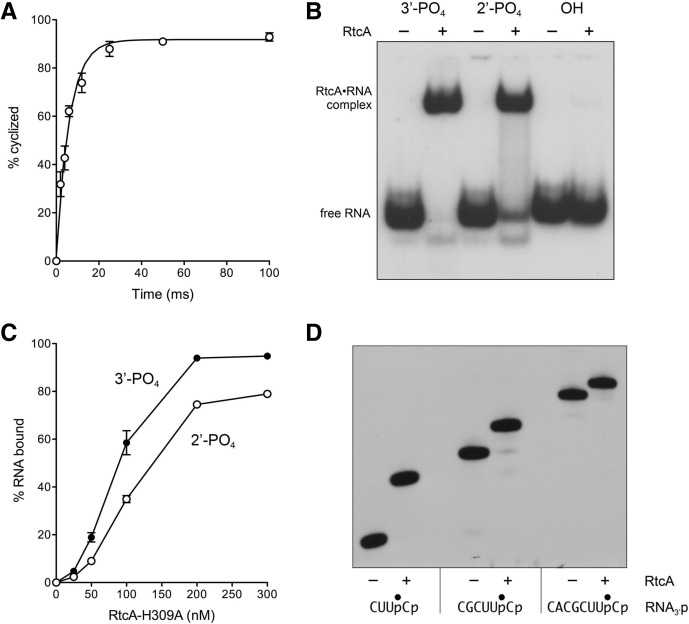
FIGURE 3.
Kinetics of 3′-phosphate cyclization, binding of RtcA to 3′-phosphate and 2′-phosphate RNAs, and minimized RNA substrate for cyclization. (A) Kinetics. Reaction mixtures containing 50 mM Tris-HCl (pH 7.4), 2 mM DTT, 20 nM 32P-labeled 20-mer RNA3′p, and 200 nM RtcA were incubated at 22°C. The extent of 3′-phosphate cyclization is plotted as a function of reaction time. Each datum is the average of three separate experiments ±SEM. A nonlinear regression curve fit of the data to a one-phase association (executed in Prism) is shown. (B) RNA binding. Reaction mixtures containing 50 mM Tris-HCl (pH 7.4), 1 mM DTT, 20 nM 32P-labeled 20-mer RNA3′p (3′-PO4) or RNA2′p (2′-PO4) or RNAOH (OH) substrate, and either no enzyme or 300 nM of RtcA-H309A (where indicated by +) were incubated for 20 min at 37°C. The mixtures were placed on ice, adjusted to 10% glycerol and 0.04% Triton-X100, and then analyzed by native PAGE. The 32P-labeled free RNAs and RtcA•RNA complexes were visualized by autoradiography. (C) Reaction mixtures containing 50 mM Tris-HCl (pH 7.4), 1 mM DTT, 20 nM of a 20-mer RNA3′p (3′-PO4) or RNA2′p (2′-PO4) substrate, and RtcA-H309A as indicated were incubated for 20 min at 37°C. The extents of RtcA•RNA complex formation are plotted as a function of input RtcA. Each datum is the average of three independent RtcA titration experiments (±SEM). (D) Minimized RNA substrate. Reaction mixtures (10 µL) containing 50 mM Tris-HCl (pH 7.4), 10 mM MgCl2, 2 mM DTT, 100 µM ATP, 20 nM of 4-mer, 6-mer, or 8-mer RNA3′p substrates labeled with 32P at the penultimate phosphate, and either no enzyme (−) or 200 nM RtcA (+) were incubated for 30 min at 37°C. The reactions were quenched by adding an equal volume of 90% formamide and 50 mM EDTA, and then analyzed by urea-PAGE. An autoradiogram of the gel is shown. The nucleotide sequences of the RNA3′p strands are shown below the autoradiogram; the position of the 32P-label is denoted by •.