Abstract
Study Design Review.
Objective Postoperative oropharyngeal dysphagia is one of the most common complications following anterior cervical spine surgery (ACSS). We review and summarize recent literature in order to provide a general overview of clinical signs and symptoms, assessment, incidence and natural history, pathophysiology, risk factors, treatment, prevention, and topics for future research.
Methods A search of English literature regarding dysphagia following anterior cervical spine surgery was conducted using PubMed and Google Scholar. The search was focused on articles published since the last review on this topic was published in 2005.
Results Patients who develop dysphagia after ACSS show significant alterations in swallowing biomechanics. Patient history, physical examination, X-ray, direct or indirect laryngoscopy, and videoradiographic swallow evaluation are considered the primary modalities for evaluating oropharyngeal dysphagia. There is no universally accepted objective instrument for assessing dysphagia after ACSS, but the most widely used instrument is the Bazaz Dysphagia Score. Because dysphagia is a subjective sensation, patient-reported instruments appear to be more clinically relevant and more effective in identifying dysfunction. The causes of oropharyngeal dysphagia after ACSS are multifactorial, involving neuronal, muscular, and mucosal structures. The condition is usually transient, most often beginning in the immediate postoperative period but sometimes beginning more than 1 month after surgery. The incidence of dysphagia within one week after ACSS varies from 1 to 79% in the literature. This wide variance can be attributed to variations in surgical techniques, extent of surgery, and size of the implant used, as well as variations in definitions and measurements of dysphagia, time intervals of postoperative evaluations, and relatively small sample sizes used in published studies. The factors most commonly associated with an increased risk of oropharyngeal dysphagia after ACSS are: more levels operated, female gender, increased operative time, and older age (usually >60 years). Dysphagic patients can learn compensatory strategies for the safe and effective passage of bolus material. Certain intraoperative and postoperative techniques may decrease the incidence and/or severity of oropharyngeal dysphagia after ACSS.
Conclusions Large, prospective, randomized studies are required to confirm the incidence, prevalence, etiology, mechanisms, long-term natural history, and risk factors for the development of dysphagia after ACSS, as well as to identify prevention measures. Also needed is a universal outcome measurement that is specific, reliable and valid, would include global, functional, psychosocial, and physical domains, and would facilitate comparisons among studies. Results of these studies can lead to improvements in surgical techniques and/or perioperative management, and may reduce the incidence of dysphagia after ACSS.
Keywords: anterior approach, complication, dysphagia, oropharyngeal, cervical spine surgery
Introduction
The anterior approach to the cervical spine is used to treat numerous cervical disorders, including degenerative,1 2 3 traumatic, oncologic, inflammatory, congenital,2 4 vascular, and infectious conditions.2 The anterior approach is safe and effective and is associated with low rates of morbidity and mortality.3 4 The purpose of this article is to review the most recent literature on postoperative dysphagia after anterior cervical spine surgery (ACSS), including assessment, incidence and natural history, pathophysiology, risk factors, treatment, prevention, and topics for future research.
Oropharyngeal Dysphagia: Definition
Postoperative oropharyngeal dysphagia is one of the most common complications associated with ACSS.2 3 5 6 7 Some authors, however, consider postoperative dysphagia after ACSS an inevitable result of the surgery rather than a surgical complication.2 8
Dysphagia is a symptom indicative of an abnormality in the neural control of, or the structures involved in,9 any phase of the swallowing process,10 which involve both voluntary and involuntary/reflex responses.11 Oropharyngeal dysphagia is an impairment in the speed and/or safe delivery of food materials from entry in the mouth to the upper portion of the esophagus.12 The patient is at an increased risk of aspiration and may be unable to swallow or have trouble swallowing liquids, foods, or saliva. The condition is considered long term if it is still present more than 4 weeks after surgery.13
Normal swallowing involves more than 30 muscles and is performed up to 600 times a day.14 Dysphagia can occur during any or all of the three phases of swallowing,15 including the oral preparatory and transport phase (sucking, chewing, and moving food or liquid into the throat); the pharyngeal phase (starting the swallowing reflex, squeezing food down the throat, and closing off the airway to prevent aspiration of food or liquid or to prevent choking); and the esophageal phase (relaxing and tightening the openings at the top and bottom of the esophagus and squeezing food through the esophagus into the stomach).16 Oropharyngeal dysphagia can occur during the oral phase, the pharyngeal phase, or both,17 and the swallowing dysfunction can be divided into four categories1: an inability or excessive delay in initiating pharyngeal swallowing,2 ingestate aspiration,3 nasopharyngeal regurgitation,4 and ingestate residue within the pharyngeal cavity after swallowing.17
Clinical Signs and Symptoms
Patients who develop dysphagia after ACSS show significant alterations in swallowing biomechanics, which include increased aspiration, thickening of the pharyngeal wall, poorer pharyngeal constriction and peristalsis, prolonged transit time, reduced hyoid displacement, reduced opening of the pharyngoesophageal segment opening, and impaired epiglottic inversion.18 General clinical signs may include reflexive coughing or wet/gurgly voice during or right after swallowing; extra effort or time needed to chew or swallow; food or liquid leaking from the mouth or getting stuck in the mouth; and recurring pneumonia or chest congestion after eating.16 Dysphagia may result in weight loss and dehydration from insufficient calorie consumption; risk of aspiration can lead to aspiration pneumonia and chronic lung disease and embarrassment or isolation in social situations involving eating.16 17
Assessment
Significant dysphagia after ACSS requires prompt evaluation to exclude any potentially reversible surgical complications.19 The initial evaluation should involve plain cervical radiographs to rule out structurally induced dysphagia,19 including that caused by bone graft dislodgement, retropharyngeal abscess, or postoperative edema or hematoma.5
The clinical (or bedside) examination is usually the first step in a comprehensive evaluation and is useful for determining the need for further instrumental evaluation.16 Patient history, physical examination, X-ray (Fig. 1), direct or indirect laryngoscopy, and videoradiographic swallow evaluation (VSE) are considered the primary modalities for evaluating oropharyngeal dysphagia.17 20 The patient history can elicit the circumstances of symptom onset, duration, and progression.17 The physical examination includes assessment of oral sensation, oral reflexes, and postural abnormalities, as well as motor assessment of face, lips, tongue, palate and larynx, level of arousal, ability to follow directions, and saliva management.12 Neurologic examination should include cranial nerve testing, especially the nerves involved in swallowing (the sensory components of cranial nerves V, IX, and X, and the motor components of cranial nerves V, VII, X, XI, and XII).21 Laboratory evaluations may identify an underlying and treatable cause of the dysphagia (e.g., myasthenia gravis, inflammatory myopathies, or toxic and/or metabolic myopathies).17
Fig. 1.
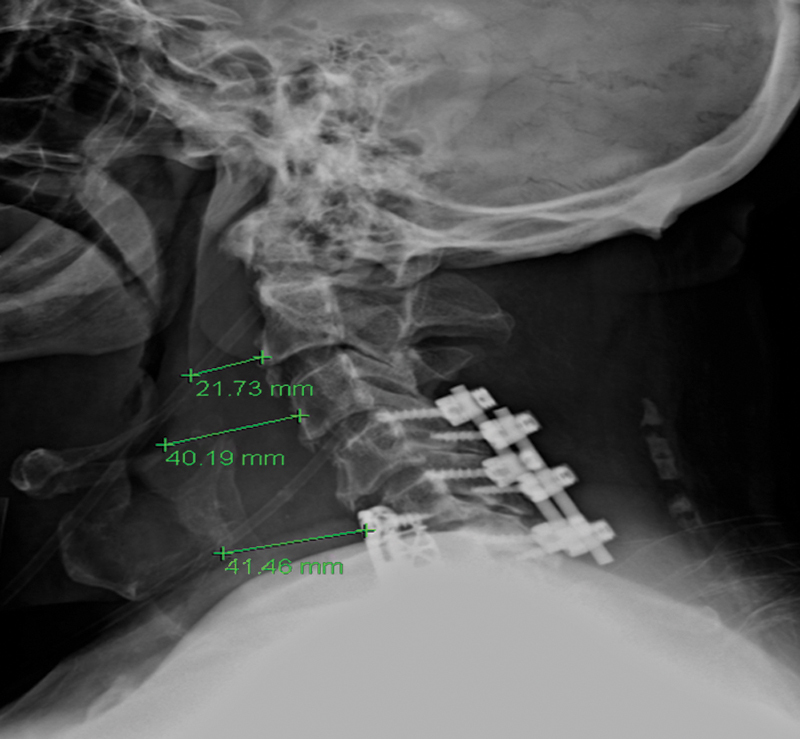
Soft tissue edema following anterior cervical discectomy and fusion (ACDF).
During a workup for dysphagia, clinicians should distinguish between the following conditions: (1) true dysphagia from globus sensation, xerostomia, or odynophagia, and whether the dysphagia is pharyngeal or esophageal; (2) a functional (motor) disorder from a structural abnormality, which can often be managed effectively by endoscopy or corrective surgery; (3) any underlying, treatable, related/causative systemic condition (e.g., extrapyramidal movement disorders); (4) the mechanics of the dysfunction (assessed using a modified barium swallow [MBS] with or without manometry to determine if the dysfunction is amenable to swallow therapy); and (5) the risk of aspiration (assessed with VSE for accurate detection of aspiration).17 22
If the underlying etiology of the dysphagia remains unclear, laboratory evaluations and various instrumental evaluations may provide additional diagnostic information. Instrumental examinations include plain X-ray (Fig. 2); videofluoroscopy (VSE) or videofluoroscopic swallowing study; endoscopy (fiberoptic endoscopic evaluation of the swallow); ultrasound to observe movement of swallowing structures; electromyography (EMG) to record electrical activity of swallowing muscles; esophageal manometry; and fiberoptic nasopharyngeal laryngoscopy.11 16 21 23 24 25
Fig. 2.
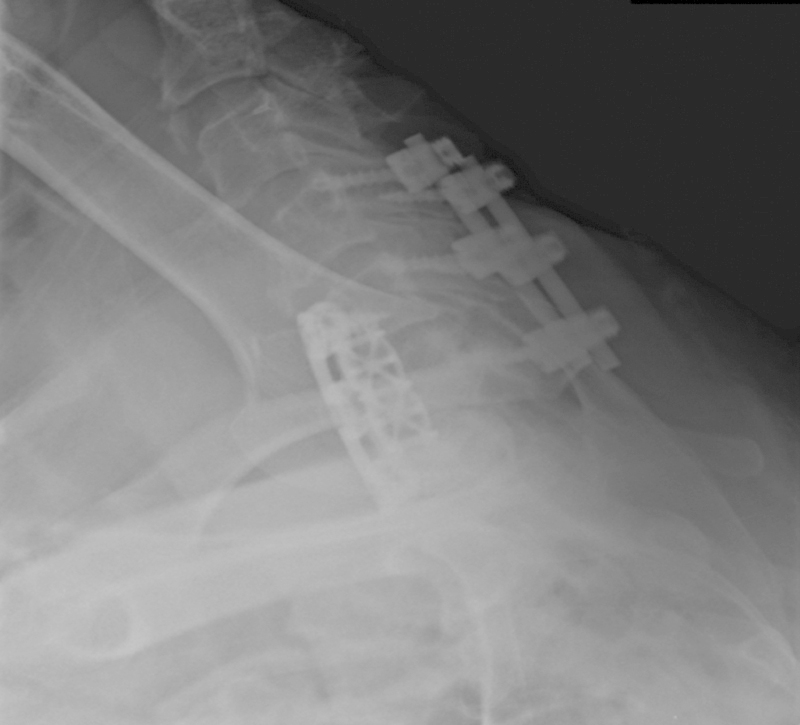
Swimmer's view shows soft tissue swelling after C5–7 anterior cervical discectomy and fusion (ACDF).
The VSE, often referred to as an MBS,17 is considered the gold standard, but it is extremely sensitive in patients undergoing ACSS.25 The VSE/MBS is usually performed by a speech pathologist along with a radiologist and provides direct videofluoroscopic imaging of the oral cavity, pharynx, and esophagus.9 22 The VSE/MBS is used to determine the presence, severity, and timing of aspiration and to detect and analyze functional impairment of the swallowing mechanism.22 A series of swallows of contrast material in varied volumes and consistencies are imaged and framed to include the oropharynx, palate, proximal esophagus, and proximal airway. Studies are recorded to permit instant replay.17 Complete approximation of the true vocal cords normally prevents movement of contrast material into the laryngeal aditus. Fig. 3 shows extravasation of contrast posterior to the esophagus at C6 with a small collection in the prevertebral space, consistent with esophageal leak. Contrast is also seen extending into the bronchi area, consistent with aspiration due to swallow dysfunction (Fig. 3).
Fig. 3.
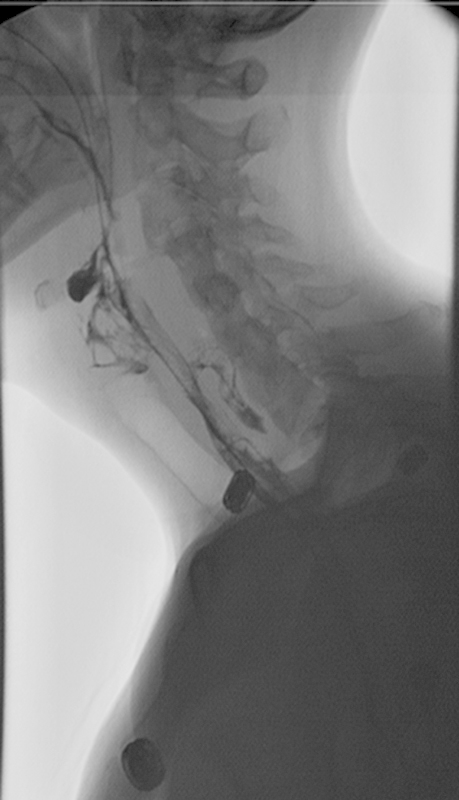
Swallow study, 6 days postoperatively. Note contrast extravasation along the posterior aspect of the esophagus at C6 with a small collection in the prevertebral space, consistent with esophageal leak. Contrast was also seen injuring the trachea extending into the bilateral main stem bronchi area, consistent with aspiration due to swallow dysfunction.
Unfortunately, there is no universally accepted objective instrument for assessing dysphagia after ACSS.26 Numerous objective instruments have been used in recent studies, including the: Bazaz Dysphagia Score27 28 29; Modified Bazaz Dysphagia Score30; World Health Organization Dysphagia Grade31; the difficulty swallowing item of the Cervical Spine Outcomes Questionnaire32; Prevertebral Soft Tissue Swelling Index20; modified Japanese Orthopaedic Association (mJOA) score for classifying cervical spondylotic myelopathy severity33; Dysphagia Numeric Rating Scale29; and plain lateral cervical radiographs.13 20 29 34 The Oswestry Neck Disability Questionnaire,32 Oswestry Disability Index,1 Short-Form 36,32 and Neck Disability Index20 are functional outcome measures that are useful in evaluating the impact of neck pain on activities.
The most widely used objective instrument for assessing dysphagia after ACSS is the Bazaz Dysphagia Score; its wide use allows for comparison of results among studies (Table 1).35 Bazaz et al created this grading system for their study because no validated grading system existed at that time. Based on telephone interviews, the patients' dysphagia symptoms were graded as none, mild, moderate, or severe. A grade of “none” indicated the patient experienced no episodes of swallowing difficulty with either liquids or solids. “Mild” indicated no difficulty with liquids and only rare difficulty with solids. “Moderate” indicated no (or rare) difficulty with liquids and occasional difficulty with specific solids such as bread or steak. “Severe” indicated no (or rare) difficulty with liquids and frequent difficulty with most solids.35 However, Skeppholm et al pointed out several drawbacks to the Bazaz Score: (1) it is clinician-administered, which may introduce a bias by the therapist who interprets the patient's condition; (2) it is oversimplified, which may result in a lack of discrepancy between patients; (3) it scores difficulties in swallowing solids worse than difficulties in swallowing liquids, when patients often experience the opposite; and (4) it has never been formally validated despite its wide use.3
Table 1. Bazaz Dysphagia Score.
| Episodes of swallowing difficulty (by patient report) | ||
|---|---|---|
| Severity of dysphagia | Liquid | Solid |
| None | None | None |
| Mild | None | Rare |
| Moderate | None or rare | Occasional (only with specific foods like bread or meat) |
| Severe | Present | Frequent (and with a majority of solids) |
Adapted from Bazaz et al, 2002.35
The objective assessments of swallowing ability, however, are often inadequate for complete diagnosis, because dysphagia is a subjective sensation of disturbance or discomfort when swallowing.3 Although physiologic instruments such as the barium swallow are valuable for determining the extent of dysfunction, their results do not correlate closely with patients' symptoms.26 Patient-reported instruments appear to be more clinically relevant and more effective in identifying dysfunction.29 36 Various patient-reported instruments used in recent literature are listed in Table 2.
Table 2. Various patient-reported instruments used in recent literature.
| Name of instrument | Notes |
|---|---|
| MDADI15 and SWAL-QOL52 | Both validated, although patients with ACSS comprise a subgroup with less severe symptoms than are usually evaluated with these two instruments3 |
| DSQ and the modified DSQ30 | An “expert opinion” validation that has good correlation with the MDADI3 |
| DDI25 | An interview for subjective complaints25 |
| VAS | For neck, arm, and iliac graft site pain1 or for dysphagia discomfort or hoarseness20 28 51 |
| Telephone interviews | Using Bazaz questions as template37; used to elicit dysphagia symptoms13 or to obtain responses to questionnaires (such as the DNRS or DSQ)36 for determining Bazaz Score36 37 |
| Patient reports of difficulty swallowing solids and/or liquids32 34 38 | Including frequency36 52 and severity of episodes,30 as well as any odynophagia (pain when swallowing)20 32 |
| Use of pain drawings, pain medication use, and patient's overall opinion of treatment success1 |
Abbreviations: ACSS, anterior cervical spine surgery; DDI, Dysphagia Disability Index; DNRS, Dysphagia Numeric Rating Scale; DSQ, Dysphagia Short Questionnaire; MDADI, MD Anderson Dysphagia Inventory; SWAL-QOL, Swallowing-Quality of Life Survey; VAS, Visual Analog Scale.
Pathoanatomy/Physiology
The causes of oropharyngeal dysphagia after ACSS are multifactorial and involve neuronal, muscular, and mucosal structures.11 28 37 38 In some cases, dysphagia can occur in the absence of any overt postoperative complications.39 Smith-Hammond et al reported that some dysphagia after ACSS is due to factors other than the anterior approach alone, because dysphagia had also been observed in posterior cervical surgery.25 Rihn et al reported that dysphagia is likely due to the anterior approach alone rather than other potential causes such as endotracheal tube cuff placement and general anesthesia. The authors postulated that dysphagia after ACSS is caused by a combination of factors, including soft tissue swelling in the neck, esophageal dysmotility, and altered sensation resulting from traction on the nerves during surgery.36
An impairment that is predominant in a specific phase of swallowing may suggest a particular etiology.39 Nanda et al reported that difficulty during the oral stage suggests damage to the hypoglossal nerve, whereas difficulty during the pharyngeal phase suggests disruption during retraction in the connections between the pharyngeal plexus and the pharyngeal muscles.19 Dysfunction during the oral preparatory/transport phase can involve reduced labial seal; reduced labial or buccal tension/tone; reduced lingual strength, range of motion, or coordination; poor labial and facial muscle function; sialorrhea or xerostomia; difficulty initiating swallowing; piecemeal swallowing; or inability to chew or propel the bolus from the mouth.11 17 Dysfunction during the pharyngeal phase can involve both sensory and motor components, such as incomplete velopharyngeal, laryngeal vestibule, or glottic closure; reduced tongue base posterior movement; reduced pharyngeal wall contraction; reduced elevation and anterior movement of the hyoid and larynx; cricopharyngeal dysfunction or weakness in the base of tongue; delay in triggering the swallow; weakness and reduced pressure in the swallow; an immediate sense of bolus holdup localized to the neck; nasal regurgitation; repeated swallowing to clear food or fluid from the pharynx; or coughing or choking during swallowing.11 17
Knowledge of the anatomy and function of both the recurrent laryngeal nerve (RLN) and the external branch of the superior laryngeal nerve (SLN) is essential in minimizing postoperative dysphagia.40 The most common nerve involved in dysphagia and aspiration encountered during ACSS is the RLN, which has different courses on each side of the neck. On the left side, the RLN branches from the vagus nerve and then passes below the aortic arch and reverses course superiorly and posteriorly as it enters the neck. The right RLN, however, branches from the vagus at the level of the subclavian artery, traverses behind the artery, and ascends into the neck. Both the left and right RLN tend to be more lateral in the lower neck and medial in the higher neck. Some patients have a nonrecurrent RLN, which enters the cricothyroid muscle without entering the chest. The inferior thyroid artery is not a reliable anatomical landmark because the RLN can pass between, anterior to, or posterior to its branches. 40 The RLN is best identified at the inferior cornu of the thyroid cartilage, then its course is traced from the trachea-esophageal groove down into the base of the neck. 40 Injury to the RLN can result in diminished closure of the glottis, as well as denervation of the inferior constrictor and cricopharyngeus muscles. Routine identification and dissection of the RLN can reduce the incidence of injury. 40 Medialization thyroplasty can be done to allow for complete glottic closure and prevention of aspiration.
The SLN has both internal and external branches. The external branch is involved in high-pitch sound and is vulnerable to injury when the superior thyroid vessels are divided. In most patients, the external SLN branch courses along the lateral surface of the inferior constrictor and terminates at the cricothyroid muscle.40 The nerve then crosses the superior thyroid artery more than 1 cm above the upper pole of the thyroid gland. The external branch of the SLN can course at the level of, below, or superior to the upper pole vessels of the thyroid. Dividing the upper pole vessels just off the thyroid capsule can minimize the chance of direct or traction injury to the SLN.40 A high cervical approach places the internal branch of the SLN at risk. Sensory innervation is usually bilateral, such that ipsilateral injury may be asymptomatic. However, bilateral injury will result in complete loss of the laryngeal cough reflex, risking aspiration pneumonia.
In revision cases, a contralateral approach is often planned. This approach allows the surgeon to dissect through less scar, which may prevent injury to the RLN where the anatomy can be obscured. Before proceeding with this approach, an ear, nose, and throat evaluation is essential to assure adequate functioning of both vocal cords. If one of the nerves is abnormal on laryngoscopy, surgery should be performed from the contralateral side.
Although the exact etiology of dysphagia after ACSS is still unknown, many authors have suggested likely causes, or ruled out various causes, based on their study results. Nanda et al reported that transient dysphagia (within 4 hours) is usually related to postoperative pharyngeal edema secondary to prolonged retraction or trauma during intubation, and delayed dysphagia (after 48 hours) is considered secondary to damage of the nerve supplying the pharyngeal muscles and is commonly attributed to forceful retraction, surgical manipulation, and aggressive use of monopolar diathermy.19
In a prospective cohort study of 92 patients who underwent anterior cervical discectomy and fusion (ACDF) at up to three levels, Papavero et al found no correlation between the amount (pressure) of intraoperative pharynx/esophagus retraction and the development of dysphagia within 5 postoperative days.30 Chin et al conducted a prospective radiographic analysis involving 64 patients who underwent ACDF, corpectomy, or both, using 2-mm plates.13 Their study was a follow-up to the Lee et al study,27 and it was the first study to use preoperative osteophyte height (not width or volume of space occupied) to determine whether dysphagia after instrumented ACSS could be caused by plate thickness or by other factors such as preoperative osteophyte height or plate location.26 The authors concluded that preoperative osteophyte height was not a factor in the development of postoperative dysphagia and that neither plate thickness of 2 mm or plate prominence of 3 to 7 mm contributed to dysphagia.13 Kepler et al conducted a prospective controlled cohort study of 43 patients who underwent one- or two-level ACDF.29 Although the authors did not include objective testing to confirm the subjective symptoms, and their use of lateral radiographs may not have provided adequate sensitivity,26 Kepler et al found that prevertebral soft tissue swelling was not associated with the development of dysphagia at any cervical level at 2 or 6 weeks postsurgery.29
The causes of oropharyngeal dysphagia can be grouped into various broad etiologic categories: (1) iatrogenic (including postsurgical muscular or neurogenic), infectious, metabolic, myopathic, neurologic, or structural (including cervical osteophytes)14 17; (2) conditions that give rise to fixed mechanical obstruction, generalized (systemic) conditions, or intrinsic functional disturbances41; (3) neuromyogenic or structural22; and (4) multifactorial, which includes collagen diseases, congenital disorders, congenital neurologic or structural malformations, medical, medications, neurologic, neurosurgical procedures, progressive neurologic disorders, structural, or radiotherapy.11 These etiologic categories of oropharyngeal dysphagia, and representative conditions in each category, are listed in Table 3. The causes of oropharyngeal dysphagia after ACSS attributed to the operative approach or the operative technique are described in Table 4.
Table 3. Causes of oropharyngeal dysphagia according to categories, with corresponding representative conditions.
| Categories of causes | Representative conditions |
|---|---|
| Collagen diseases | Scleroderma, dermatomyositis |
| Conditions that give rise to fixed mechanical obstruction | Previous surgical treatment, tumor, cervical rings or webs, radiation/radiotherapy (pharyngeal phase) |
| Congenital neurologic/structural disorders/malformations | Dysautonomia, cleft palate, cerebral palsy, muscular dystrophy |
| Iatrogenic | Medications (chemotherapy, neuroleptics, etc.); pill injury (intentional; oral preparatory phase) |
| Infectious | Botulism, diphtheria, Lyme disease, mucositis (herpetic lesions, cytomegalovirus, Candida, aphthous ulcers); syphilis (oral preparatory phase) |
| Intrinsic functional disturbances | Cricopharyngeal achalasia, Zenker diverticulum (pharyngeal phase) |
| Medical | Advanced chronic obstructive pulmonary disease, deconditioning, intubation (prolonged endotracheal), rheumatoid arthritis, some viral infections |
| Metabolic | Amyloidosis, Cushing syndrome, thyrotoxicosis, Wilson disease |
| Myopathic | Connective tissue disease (overlap syndrome), myotonic dystrophy, paraneoplastic syndromes, polymyositis, sarcoidosis |
| Neurologic | Dementia, Guillain-Barré syndrome, Huntington disease, metabolic encephalopathies, polio, postpolio syndrome, traumatic brain injury, seizure disorders, tardive dyskinesia, brainstem tumor, cerebral vascular accident |
| Neuromyogenic | Myopathies (inflammatory, metabolic), parkinsonism, head trauma, stroke (oral preparatory phase and pharyngeal phase) |
| Progressive neurologic disorders | Dystonia, progressive supranuclear palsy, oculopharyngeal dystrophy, myasthenia gravis, amyotrophic lateral sclerosis, multiple sclerosis, Parkinson's disease (oral preparatory phase and pharyngeal phase) |
| Neurosurgical procedures | Aneurysm clippings, anterior cervical spine surgery; resection of tumor |
| Structural | Extrinsic compression, cervical osteophytes, scar tissue (oral/pharyngeal), stenosis (postsurgical/radiation/idiopathic), cricopharyngeal bar, skeletal abnormalities (pharyngeal phase) |
Table 4. Causes of oropharyngeal dysphagia according to operative approach and operative technique.
| Approach/technique | Possible resulting condition |
|---|---|
| Operative approach (anterior) | |
| Dissection or retraction | Damage of the aerodigestive pathway38; muscle and serosa injuries and edema20; tissue damage with subsequent edema13; bruising or laceration of tissues38
SLN injury,29 most at risk with surgery involving C3-C4,5 15 42 which can cause laryngeal sensory impairment5 Injuries to the pharyngeal plexus or vagus nerve, glossopharyngeal nerve, or hypoglossal nerve (most at risk with surgery at or above C3)15 34 Dysfunction of the pharyngeal plexus, which affects the motility of the visceral wall30 |
| Dissection or retraction of the longus colli muscle | Muscle and subperiosteal bleeding; prevertebral soft tissue swelling20 |
| Retraction | Denervation of the pharyngeal plexus35 (involving the glossopharyngeal nerve and the pharyngeal branch of the vagus nerve)29 |
| Excessive or prolonged retraction | Dysphagia15
25
28
29
38
39
52
Esophageal edema,25 impingement,13 ischemia,28 36 55 56 denervation,5 reperfusion injury36 Posterior pharyngeal wall edema, preventing a full epiglottic deflection30 Soft tissue fibrosis28; soft tissue swelling5 30 36 39; scar tissue formation5 19 |
| Significant tension during lateralization of the larynx (RLN most at risk with surgery involving C3–C4 and C5–T1) | RLN injury, which can cause vocal fold paresis or paralysis42 |
| RLN stretch injury and/or RLN compression injury from ET cuff compression | RLN palsy, which can cause vocal fold paresis or paralysis42 |
| Use of rh-BMP-2 | Early local inflammatory response to rh-BMP-2 (dose-related)48 |
| Concurrent intraoperative traction on both the RLN and pharyngeal plexus36 | RLN injury |
| Other aspects of operative approach | |
| Direct esophageal injury | Impaired opening of the upper esophageal sphincter30
Localized denervation of portions of the esophagus and hypopharynx13 28 Pharyngeal wall ischemia55 56 |
| Hemostatic or coagulopathy | Hematoma formation19 23 |
| Operative technique | |
| Use of instrumentation | Any mechanical irritation or impingement against the esophagus27
Differences in postoperative cervical kyphotic-lordotic deformity28 |
| Thickness or anterior profile of anterior cervical plates and instrumentation | Irritation and inflammation13 28 |
| Plate on the esophagus | Mass effect29 |
| Use of graft | Graft (implant) protrusion,19 34 graft extrusion or cord compression13 |
| Improper halo or collar positioning | Cervical hyperextension23 |
Abbreviations: ET, endotracheal; rhBMP-2, recombinant human bone morphogenetic protein-2; RLN, recurrent laryngeal nerve; SLN, superior laryngeal nerve.
Incidence, Prevalence, and Natural History
Dysphagia is the most common postoperative patient complaint following ACSS and is usually a transient condition.36 42 It most often begins in the immediate postoperative period, but may begin more than 1 month after surgery.13 39 The incidence of dysphagia within 1 week after ACSS varies widely in the literature,3 25 from 1 to 79%.8 26 35 36 43 44 45 During the intermediate to longer-term postoperative period (1 to 6 weeks), the reported incidence is 28 to 57%.5 25 27 37 46 Higher incidence rates of dysphagia tend to be reported in prospective studies and with patient self-reports,3 25 26 compared with rates recorded in chart notes and in previous retrospective studies.28 Danto et al noted that repeated questioning of patients about their dysphagia symptoms will likely result in a higher incidence rate compared with rates obtained by patient self-reports.8 46 Objective measurements may underestimate the incidence.3 25
The wide variance in published incidence rates can be attributed to variations in surgical techniques, extent of surgery, and the size of the implant used, as well as variations in definitions and measurements of dysphagia, the time intervals of postoperative evaluations,3 8 13 15 37 and the relatively small sample sizes used in published studies.8 These variations are evident in the accompanying table (Table 5). Smith-Hammond et al reported in 2004 that most studies on dysphagia after ACSS up to that time had significant limitations: most were retrospective and unable to account for any preoperative swallowing difficulties; many relied on patient self-report, which was considered not completely reliable; many used different definitions of dysphagia; some used a right- rather than left-sided approach, which may have affected incidence rates; and, finally, prior studies did not include control patients.25
Table 5. Summary of incidence and prevalence rates.
| Author, year | No. of patients | Assessment tool | Immediate postoperative (<2 wk) | 2 wk: incidence | 1 mo–6 wk | 8 wk–3 mo | 6 mo | 12 mo | 24 mo |
|---|---|---|---|---|---|---|---|---|---|
| Lee et al, 200527 | 156 | Bazaz Dysphagia Score by telephone | x | x | Incidence: 48.9%; prevalence: x | Incidence: x; prevalence: 37.0% | Incidence: x; prevalence: 20.9% | Incidence: x; prevalence: 15.4% | Incidence: x; prevalence: 11.4% |
| Riley et al, 200532 | 454 | CSOQ self-report of swallowing impairment | x | x | x | Incidence: 28.2%; prevalence: x | Incidence: 6.8%; prevalence: 21.5% | x | Incidence: 7%; prevalence: 21.3% |
| Yue et al, 200534 | 74 | Bazaz Dysphagia Score | x | x | x | x | Incidence: x; prevalence at undefined “early postoperative period”: 45.9% | x | Incidence: x; prevalence at 5+ y: 35.2% |
| Chin et al, 200713 | 64 | Telephone assessment of subjective swallowing difficulties | 34% | x | x | Incidence: x; prevalence at >4 wk: 17% | x | x | x |
| Lee et al, 200737 | 310 | Bazaz Dysphagia Score | x | x | Incidence: 54%; prevalence: x | Incidence: x; prevalence: 33.6% | Incidence: x; prevalence: 18.6% | Incidence: x; prevalence: 15.2% | Incidence: x; prevalence: 13.6% |
| Papavero et al, 200730 | 92 | Modified dysphagia questionnaire | At < 5 d postoperatively: 49.3% | x | x | x | x | x | x |
| Tervonen et al, 200744 | 114 | VAS for all; transoral endoscopic evaluation and videofluorography for some | 69% | x | x | Incidence: x; prevalence at 3–9 mo: 27% | Incidence: x; prevalence at 3–9 mo: 27% | x | x |
| Vaidya et al, 200748 | 18 | Bazaz Dysphagia Score | 56% | 39.0% | Incidence: 22%; prevalence: x | x | x | x | Incidence: x; prevalence: 22.0% |
| McAfee et al, 201028 | 251 | Bazaz Dysphagia Score; hoarseness VAS | x | x | Incidence: 57.8%; prevalence: x | Incidence: x; prevalence: 38.5% | Incidence: x; prevalence: 27.2% | Incidence: x; prevalence: 28.9% | Incidence: x; prevalence: 27.6% |
| Riley et al, 2010 (systematic review)26 | N/A | N/A | 33.1% (range 1–79%) | x | Incidence: x; prevalence: 53.2% (range 50–56%) | Incidence: x; prevalence: 31.6% (range 28–37%) | Incidence: x; prevalence: 19.8% (range 8–22%) | Incidence: x; prevalence: 16.8% (range 13–21%) | Incidence: x; prevalence: 12.9% (range 11–14%) |
| Pattavilakom and Seex, 201138 | 26 | Self-assessment of sore throat, dysphagia, and dysphonia quantified with Likert scale | at 24 h: 46%; at 1 wk: 32% | x | Incidence: 5%; prevalence: x | x | x | x | x |
| Rihn et al, 201136 | 38 | Bazaz Dysphagia Score derived from answers to questionnaire and numeric rating scale | x | 71.0% | Incidence: x; prevalence: 26.0% | Incidence: x; prevalence: 8.0% | x | x | x |
| Fehlings et al, 201233 | 302 | Included only clinically apparent and significant forms | x | x | Incidence at < 30 d: 3%; prevalence: x | x | x | x | x |
| Kalb et al, 20124 | 249 | DDI (score of ≥ 30) | x | x | Incidence: 88.4%; prevalence: x | Incidence: x; prevalence: 29.6% | Incidence: 10.8%; prevalence: 7.4% | x | x |
Abbreviations: CSOQ, Cervical Spine Outcomes Questionnaire; DDI, Dysphagia Disability Index; N/A, not applicable; VAS, Visual Analog Scale.
Since the last review on postoperative dysphagia by Lee et al,47 several studies have provided more information regarding the incidence and natural history of oropharyngeal dysphagia after ACSS. Riley et al reported on dysphagia rates in 454 patients who underwent ACSS and were enrolled in the multicenter Cervical Spine Research Society Outcomes Study between 1998 and 2001.32 In this retrospective observational study, the incidence of post-ACSS dysphagia was 28.2, 6.8, and 7.0% at 3, 6, and 24 months, respectively. At both 6 and 24 months, the prevalence rate of persistent swallowing dysfunction was 21%.32
Five years later, in a systematic review of prospective studies on dysphagia after ACSS, Riley et al reported that incidence rates declined steadily over time after surgery and plateaued at a rate of 13 to 21% at 1 year.26 The average incidence rates assessed at different times after ACSS were as follows: 53.2% at 1 month27 35 37; 31.6% at 2 to 4 months27 32 35 37; 19.8% at 6 months27 32 35 37 48; 16.8% at 12 months27 32 35 37; and 12.9% at 24 months.27 37 The overall incidence rate they reported of 30% at 3 months is considered a representative benchmark that is consistent with other published reports.26 28
Pattavilakom and Seex conducted a prospective, randomized, controlled study of 26 patients over an 18-month period. They found a cumulative incidence of moderate dysphagia of 45% at 24 hours and 5% at 28 days.38 Rihn et al conducted a prospective controlled study of 94 patients, 38 of whom had primary one- or two-level ACDF for a degenerative condition. At 2-week follow-up, 71% of those 38 patients reported some degree of dysphagia according to the Bazaz Scale. This rate decreased to 26 and 8% at 6 weeks and 12 weeks postoperatively, respectively.36 Fehlings et al conducted a prospective multicenter study evaluating perioperative complications (< 30 days) in 302 patients treated for cervical spondylotic myelopathy. Although the rate of postoperative dysphagia was similar in both the anterior- and posterior-only groups (3%), the rate was significantly higher for combined anteroposterior procedures (21%).33 Kalb et al retrospectively analyzed 249 patients who underwent ACSS for cervical spondylotic disease. Only 10.8% (27/249) developed dysphagia in the first 6 postoperative months. In these 27 patients, dysphagia was assessed in 88.4, 29.6, and 7.4% at 6 weeks and 3 and 6 months, respectively. By 12 months, dysphagia had resolved in all cases.4
The rate of clinically significant dysphagia after ACSS depends on how the severity and duration of symptoms are classified.33 Both the incidence and severity of dysphagia after ACSS are high in the early postoperative period but decrease over time.36 Most cases of dysphagia are mild and transient, resolving gradually within 3 months.7 25 34 36 Without any treatment, most cases of postoperative dysphagia resolve within 12 months.49 In only 5 to 7% of cases of dysphagia after ACSS, symptoms are still present 6 to 24 months after surgery.3 25 32 34 39 Some patients describe symptoms of dysphagia years after their surgery. Yue et al reported a 15% rate of still-significant dysphagia 5 years after ACSS; however, the majority of these patients believed the positive effects of their ACSS outweighed their persistent dysphagia.34 The predominant cause of prolonged dysphagia appears related to the increased thickness of the posterior pharyngeal wall above the upper esophageal sphincter.18
Riley et al reported a prevalence rate of 21% at both 6 and 24 months,32 which contradicts previous and current reports of prevalence decreasing sharply over the first 12 months.4 23 35 Lee et al reported overall prevalence rates of dysphagia slowly decreasing over time: 54.0% at 1 month; 33.6% at 2 months; 18.6% at 6 months; 15.2% at 1 year; and 13.6% at 2 years.37 At that 2-year mark, dysphagia was reported more frequently in women, in revision surgery, with use of hardware, and in surgery at three or more levels.37
Risk Factors
There have been numerous attempts to delineate the risk factors associated with the development of oropharyngeal dysphagia after ACSS. Various authors have investigated possible correlations between dysphagia after ACSS and demographic factors (e.g., age, gender, use of tobacco/alcohol, hypertension, diabetes) and/or surgical factors (e.g., operative time, use of instrumentation, plate design, extent of intraoperative retraction, endotracheal tube cuff pressure, use of steroids, numbers of levels, revision versus primary surgery; Fig. 4). However, the wide variety of results precludes any firm conclusions.4 26 Controlling for confounding factors can be challenging. Danto et al noted that attempting to prove that plates are a risk factor for postoperative dysphagia may be difficult, because the surgery required to insert the plates may be a confounding factor.8
Fig. 4.

Dysphagia following extrusion of bone graft. The patient underwent revision surgery and an anteroposterior fusion, with resolution of dysphagia symptoms several months after the second surgery.
The factors most commonly reported as being associated with an increased risk of oropharyngeal dysphagia after ACSS are: a greater number of levels operated (Fig. 5),4 8 26 32 35 36 37 48 50 gender (female),8 26 30 35 37 51 52 increased operative time,4 13 32 33 36 and older age (usually > 60 years).4 25 33 These and other risk factors are listed in Table 6. It is important to note that these same risk factors were reported as not being associated with an increased risk of oropharyngeal dysphagia after ACSS in various other studies.1 4 8 13 15 23 25 27 29 30 32 33 34 35 36 37 48 52 53 54
Fig. 5.
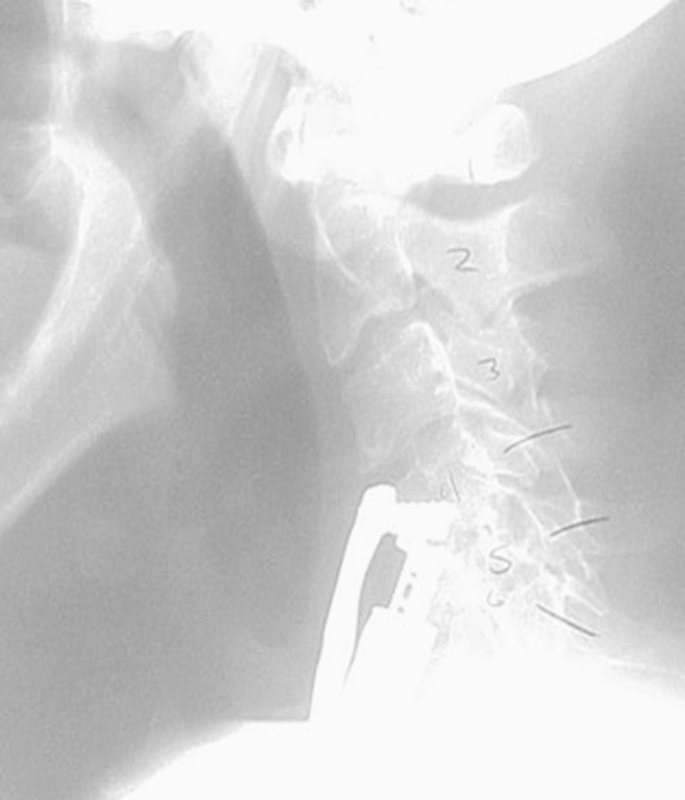
Dysphagia following collapse of long-segment construct.
Table 6. The factors most commonly reported as associated with an increased risk of oropharyngeal dysphagia after ACSS.
| Age (older),33 age > 60 y4 25 |
| ACDF versus disk replacement/arthroplasty28 50 |
| Blood loss > 300 mL32 |
| Gender (female)8 26 30 35 37 51 52 |
| Use of plating29; shorter plate constructs,13 larger and less smooth plate27 |
| Operative levels (higher versus lower),13 35 highest operative level at C3–4 versus C5–7,50 operative levels at C4–5 and C5–64 |
| Number of levels operated (more)4 8 26 32 35 36 37 48 50 |
| Increased operative time,4 32 33 36 operative time > 175 min13 |
| Excessive or prolonged esophageal retraction pressure13 15 29 |
| Revision surgery37 |
| Smoking52 |
| Prevertebral soft tissue swelling,32 swelling in the early postoperative period (1–2 mo)37 |
| Use of rh-BMP-248 |
| Soft tissue injury29 |
| Longer duration of current pain since first pain episode32; higher preoperative neck pain rating scores32 |
| Higher average intraluminal pressure throughout surgery and lower average mucosal perfusion15 |
| Scar tissue formation on a less smooth plate surface27; scar tissue amount at 1–2 y postoperatively37 |
| Highest level of plate at C3 versus C4 and below13 |
Abbreviations: ACDF, anterior cervical discectomy and fusion; ACSS, anterior cervical spine surgery; rhBMP-2, recombinant human bone morphogenetic protein-2.
Factors that were found to not be associated with an increased risk of dysphagia after ACSS include: headache at presentation32; type of incision (transverse, longitudinal, oblique)4; height of preoperative osteophytes and height of plate compared with height of preoperative osteophytes13; graft nonunion, malunion, or subsidence32 34; implant loosening and/or breakage32 34; pressure on esophageal walls exceeding mucosal perfusion pressure and 70% decrease in esophageal wall perfusion55 56; intubation8 25 52 or difficult intubation52; severity of myelopathy25 33; osteoarthritis25; alcohol/ substance abuse25; and body mass index.15 33 36 52
Rehabilitation Management and Treatment
The primary treatment interventions for oropharyngeal dysphagia are behavioral, involving postural changes, sensory input enhancements, swallowing maneuvers, voluntary controls in effort exerted during swallow, and/or diet modifications.11 The goals of treatment are to maximize food transfer and minimize or prevent aspiration.21 22
The prognosis for patients with dysphagia is affected by any complications that may develop from the condition, including pneumonia, dehydration, and malnutrition.50 The patient with dysphagia can learn various compensatory strategies for facilitating the safe and effective passage of bolus material. These strategies include: (1) modifying diet: controlling bolus size or texture,12 18 avoiding certain foods25; (2) heightening sensory input prior to or during swallowing11; (3) applying voluntary control to the swallow (breath holding, effortful swallow)11; (4) protecting the airway with postural adjustments to reduce risk of aspiration (e.g., chin tuck, head tilt, head rotation, head lift, lying down)11 12 18 25; and (5) doing exercises to strengthen weak facial muscles, to improve range of oral or pharyngeal structural movement, and/or to improve coordination.11 12 25 If the patient is still unable to swallow safely despite these rehabilitation strategies, then medical or surgical intervention may be necessary. An injection of temporary augmentation material allows immediate symptom relief with increased function and better swallowing during recovery.42 Vocal cord medialization and devices such as palatal lifts can also be used to reduce aspiration risk.12 A temporary feeding tube may be needed in cases where aspiration risk cannot be reduced and/or nutritional needs cannot be met.5 12
Prevention
Certain intraoperative and postoperative techniques may decrease the incidence and/or severity of oropharyngeal dysphagia after ACSS. These techniques are listed in Table 7.
Table 7. Intraoperative and postoperative techniques that may decrease the incidence and/or severity of oropharyngeal dysphagia after ACSS.
| Use preoperative tracheal/esophageal traction exercise57 |
| Improve surgical techniques or make changes in perioperative management30 |
| Place retractor blades cautiously under the longus colli muscles39 |
| Release the endotracheal tube cuff and reinflate it after retractor placement to minimize pressure-related damage to the RLN58 59 |
| Decrease endotracheal tube cuff pressure to 20 mm Hg to improve patient comfort following ACSS51 |
| Limit operative time to < 175 min |
| Use smaller and smoother plates27; use low-profile plates (keep the cervical plate flush against the anterior vertebral surface by burring down the preoperative osteophytes, contouring lordosis into the plate to avoid plate prominence > 7 mm, and using larger incisions for easy visualization)13 |
| Utilize a team approach during surgery: head and neck surgeon providing the exposure and neurosurgeon performing the procedures42 |
| Ensure knowledge of normal and aberrant courses of the SLN and RLN42 |
| Use low-dose oral steroids (methylprednisolone) perioperatively in selected patients to minimize neck/airway swelling and dysphagia in the acute period1 20 24 |
| Use a retropharyngeal local steroid (triamcinolone 40 mg) to control local inflammatory response thus reduce PSTS and airway swelling20 |
| Involve speech pathologists and otolaryngologists in the postsurgery evaluation, especially in cases of high risk19 25 39 |
| Introduce laryngeal diagnostic techniques and voice management early42 |
Abbreviations: ACSS, anterior cervical spine surgery; PSTS, prevertebral soft tissue swelling; RLN, recurrent laryngeal nerve; SLN, superior laryngeal nerve.
Suggestions for Future Research
Future research is required to confirm the incidence, prevalence, mechanisms, long-term natural history, and risk factors of the development of dysphagia after ACSS,8 as well as to identify recommendations for prevention of the condition. Long-term, large, prospective randomized studies1 20 25 28 32 34 may provide a more clinically relevant perspective than studies based on an administrative database.2 Future studies should control for potential confounders and randomize patients, type of surgery, use of instrumentation, and number of levels.37 A blinded assessment of postoperative dysphagia is important32 as well as long-term follow-up.36 A universal outcome measurement of dysphagia after ACSS is needed that is specific, reliable, and valid4 28 36 37 52 and would facilitate comparisons among studies.26 The ideal assessment instrument would be patient self-reported and would include global, functional, psychosocial, and physical domains.26
Topics of future studies on the causes of dysphagia after ACSS include: obtaining direct evidence that plates cause dysphagia13 32; investigating the effects of endotracheal cuff pressure, applied retraction pressure, and type of surgical fusion device8; evaluating the nature and causes of soft tissue changes (inflammation, hemorrhage, or other traumatic events during surgery)50; including the width or volume of the space occupied by the osteophyte, not just the height of the osteophyte13; investigating the effect of decreased mucosal perfusion15; analyzing the relationship between dysphagia and retraction time50; and investigating whether mechanical retraction disrupts the esophageal neural supply or induces a state of dysmotility.39 Topics for future studies on the prevention of dysphagia after ACSS include: identifying the ideal carrier for a local steroid20; determining the optimum magnitude and duration of pressure applied to the tissues38; exploring the role of intraoperative EMG in detecting laryngeal nerve injury due to stretching or sectioning8; examining the effect of intermittent versus static retraction15; and determining the effectiveness of neuromuscular electrical stimulation on the condition.12 21 Results of these studies can lead to improvements in surgical techniques and/or perioperative management and a better understanding of the impact of dysphagia symptoms on outcome as well as effective treatment measures and may reduce the incidence of dysphagia after ACSS.26 30
Footnotes
Disclosures None
References
- 1.Buttermann G R. Prospective nonrandomized comparison of an allograft with bone morphogenic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J. 2008;8:426–435. doi: 10.1016/j.spinee.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Campbell P G, Yadla S, Malone J. et al. Early complications related to approach in cervical spine surgery: single-center prospective study. World Neurosurg. 2010;74:363–368. doi: 10.1016/j.wneu.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 3.Skeppholm M, Ingebro C, Engström T, Olerud C. The Dysphagia Short Questionnaire: an instrument for evaluation of dysphagia: a validation study with 12 months' follow-up after anterior cervical spine surgery. Spine (Phila Pa 1976) 2012;37:996–1002. doi: 10.1097/BRS.0b013e31823a7a5b. [DOI] [PubMed] [Google Scholar]
- 4.Kalb S, Reis M T, Cowperthwaite M C. et al. Dysphagia after anterior cervical spine surgery: incidence and risk factors. World Neurosurg. 2012;77:183–187. doi: 10.1016/j.wneu.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Daniels A H, Riew K D, Yoo J U. et al. Adverse events associated with anterior cervical spine surgery. J Am Acad Orthop Surg. 2008;16:729–738. doi: 10.5435/00124635-200812000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Patel N P, Wolcott W P, Johnson J P. et al. Esophageal injury associated with anterior cervical spine surgery. Surg Neurol. 2008;69:20–24, 24. doi: 10.1016/j.surneu.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Kasimatis G B, Panagiotopoulos E, Gliatis J, Tyllianakis M, Zouboulis P, Lambiris E. Complications of anterior surgery in cervical spine trauma: an overview. Clin Neurol Neurosurg. 2009;111:18–27. doi: 10.1016/j.clineuro.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Danto J, DiCapua J, Nardi D. et al. Multiple cervical levels: increased risk of dysphagia and dysphonia during anterior cervical discectomy. J Neurosurg Anesthesiol. 2012;24:350–355. doi: 10.1097/ANA.0b013e3182622843. [DOI] [PubMed] [Google Scholar]
- 9.American College of Radiology. ACR Appropriateness Criteria®: Dysphagia Available at: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/Dysphagia.pdf. Accessed February 28, 2013
- 10.National Institute on Deafness and Other Communication Disorders (National Institutes of Health). Dysphagia. NIH Publication No. 10–4307. Bethesda, MD: NIH; October 2010 Available at: http://www.nidcd.nih.gov/health/voice/Pages/dysph.aspx. Accessed February 27, 2013
- 11.Logemann J A, Larsen K. Oropharyngeal dysphagia: pathophysiology and diagnosis for the anniversary issue of Diseases of the Esophagus. Dis Esophagus. 2012;25:299–304. doi: 10.1111/j.1442-2050.2011.01210.x. [DOI] [PubMed] [Google Scholar]
- 12.Flanagan S Disorders of Language, Speech and Swallowing. AAPMR American Academy of Physical Medicine and Rehabilitation: PM&R Knowledge NOW; 2012 Available at: http://now.aapmr.org/cns/complications/Pages/Disorders-of-Language,-Speech-and-Swallowing.aspx. Accessed January 14, 2013
- 13.Chin K R, Eiszner J R, Adams S B Jr. Role of plate thickness as a cause of dysphagia after anterior cervical fusion. Spine (Phila Pa 1976) 2007;32:2585–2590. doi: 10.1097/BRS.0b013e318158dec8. [DOI] [PubMed] [Google Scholar]
- 14.Lembo A J. Waltham, MA: UpToDate; 2013. Pathogenesis and clinical manifestations of oropharyngeal dysphagia. [Google Scholar]
- 15.Mendoza-Lattes S, Clifford K, Bartelt R, Stewart J, Clark C R, Boezaart A P. Dysphagia following anterior cervical arthrodesis is associated with continuous, strong retraction of the esophagus. J Bone Joint Surg Am. 2008;90:256–263. doi: 10.2106/JBJS.G.00258. [DOI] [PubMed] [Google Scholar]
- 16.American Speech-Language-Hearing Association. Swallowing disorders (dysphagia) in adults. 2013 Available at: http://www.asha.org/public/speech/swallowing/Swallowing-Disorders-in-Adults/. Accessed January 15, 2013
- 17.Cook I J, Kahrilas P J. AGA technical review on management of oropharyngeal dysphagia. Gastroenterology. 1999;116:455–478. doi: 10.1016/s0016-5085(99)70144-7. [DOI] [PubMed] [Google Scholar]
- 18.Leonard R, Belafsky P. Dysphagia following cervical spine surgery with anterior instrumentation: evidence from fluoroscopic swallow studies. Spine (Phila Pa 1976) 2011;36:2217–2223. doi: 10.1097/BRS.0b013e318205a1a7. [DOI] [PubMed] [Google Scholar]
- 19.Nanda A, Rudrappa S, Vannemreddy P. New York, NY: Oxford University Press; 2006. Iatrogenic complications of spine surgery; pp. 760–761. [Google Scholar]
- 20.Lee S H, Kim K T, Suk K S, Park K J, Oh K I. Effect of retropharyngeal steroid on prevertebral soft tissue swelling following anterior cervical discectomy and fusion: a prospective, randomized study. Spine. 2011;36:2286–2292. doi: 10.1097/BRS.0b013e318237e5d0. [DOI] [PubMed] [Google Scholar]
- 21.Lembo A J. Waltham, MA: UpToDate; 2013. Diagnosis and treatment of oropharyngeal dysphagia. [Google Scholar]
- 22.Cook I J. Diagnostic evaluation of dysphagia. Nat Clin Pract Gastroenterol Hepatol. 2008;5:393–403. doi: 10.1038/ncpgasthep1153. [DOI] [PubMed] [Google Scholar]
- 23.Frempong-Boadu A, Houten J K, Osborn B. et al. Swallowing and speech dysfunction in patients undergoing anterior cervical discectomy and fusion: a prospective, objective preoperative and postoperative assessment. J Spinal Disord Tech. 2002;15:362–368. doi: 10.1097/00024720-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Pedram M, Castagnera L, Carat X, Macouillard G, Vital J M. Pharyngolaryngeal lesions in patients undergoing cervical spine surgery through the anterior approach: contribution of methylprednisolone. Eur Spine J. 2003;12:84–90. doi: 10.1007/s00586-002-0495-6. [DOI] [PubMed] [Google Scholar]
- 25.Smith-Hammond C A, New K C, Pietrobon R, Curtis D J, Scharver C H, Turner D A. Prospective analysis of incidence and risk factors of dysphagia in spine surgery patients: comparison of anterior cervical, posterior cervical, and lumbar procedures. Spine (Phila Pa 1976) 2004;29:1441–1446. doi: 10.1097/01.brs.0000129100.59913.ea. [DOI] [PubMed] [Google Scholar]
- 26.Riley L H III Vaccaro A R Dettori J R Hashimoto R Postoperative dysphagia in anterior cervical spine surgery Spine (Phila Pa 1976) 201035(9, Suppl):S76–S85. [DOI] [PubMed] [Google Scholar]
- 27.Lee M J, Bazaz R, Furey C G, Yoo J. Influence of anterior cervical plate design on Dysphagia: a 2-year prospective longitudinal follow-up study. J Spinal Disord Tech. 2005;18:406–409. doi: 10.1097/01.bsd.0000177211.44960.71. [DOI] [PubMed] [Google Scholar]
- 28.McAfee P C, Cappuccino A, Cunningham B W. et al. Lower incidence of dysphagia with cervical arthroplasty compared with ACDF in a prospective randomized clinical trial. J Spinal Disord Tech. 2010;23:1–8. doi: 10.1097/BSD.0b013e31819e2ab8. [DOI] [PubMed] [Google Scholar]
- 29.Kepler C K, Rihn J A, Bennett J D. et al. Dysphagia and soft-tissue swelling after anterior cervical surgery: a radiographic analysis. Spine J. 2012;12:639–644. doi: 10.1016/j.spinee.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 30.Papavero L, Heese O, Klotz-Regener V, Buchalla R, Schröder F, Westphal M. The impact of esophagus retraction on early dysphagia after anterior cervical surgery: does a correlation exist? Spine (Phila Pa 1976) 2007;32:1089–1093. doi: 10.1097/01.brs.0000261627.04944.cf. [DOI] [PubMed] [Google Scholar]
- 31.Anderson P A, Sasso R C, Riew K D. Comparison of adverse events between the Bryan artificial cervical disc and anterior cervical arthrodesis. Spine (Phila Pa 1976) 2008;33:1305–1312. doi: 10.1097/BRS.0b013e31817329a1. [DOI] [PubMed] [Google Scholar]
- 32.Riley L H III, Skolasky R L, Albert T J, Vaccaro A R, Heller J G. Dysphagia after anterior cervical decompression and fusion: prevalence and risk factors from a longitudinal cohort study. Spine (Phila Pa 1976) 2005;30:2564–2569. doi: 10.1097/01.brs.0000186317.86379.02. [DOI] [PubMed] [Google Scholar]
- 33.Fehlings M G, Smith J S, Kopjar B. et al. Perioperative and delayed complications associated with the surgical treatment of cervical spondylotic myelopathy based on 302 patients from the AOSpine North America Cervical Spondylotic Myelopathy Study. J Neurosurg Spine. 2012;16:425–432. doi: 10.3171/2012.1.SPINE11467. [DOI] [PubMed] [Google Scholar]
- 34.Yue W M, Brodner W, Highland T R. Persistent swallowing and voice problems after anterior cervical discectomy and fusion with allograft and plating: a 5- to 11-year follow-up study. Eur Spine J. 2005;14:677–682. doi: 10.1007/s00586-004-0849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bazaz R, Lee M J, Yoo J U. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine (Phila Pa 1976) 2002;27:2453–2458. doi: 10.1097/00007632-200211150-00007. [DOI] [PubMed] [Google Scholar]
- 36.Rihn J A, Kane J, Albert T J, Vaccaro A R, Hilibrand A S. What is the incidence and severity of dysphagia after anterior cervical surgery? Clin Orthop Relat Res. 2011;469:658–665. doi: 10.1007/s11999-010-1731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee M J, Bazaz R, Furey C G, Yoo J. Risk factors for dysphagia after anterior cervical spine surgery: a two-year prospective cohort study. Spine J. 2007;7:141–147. doi: 10.1016/j.spinee.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 38.Pattavilakom A Seex K A Results of a prospective randomized study comparing a novel retractor with a Caspar retractor in anterior cervical surgery Neurosurgery 201169(2, Suppl Operative):ons156–ons160., discussion ons160 [DOI] [PubMed] [Google Scholar]
- 39.Lee S K, Lee G Y, Wong G T. Prolonged and severe dysphagia following anterior cervical surgery. J Clin Neurosci. 2004;11:424–427. doi: 10.1016/j.jocn.2003.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Chen H. How to manage voice changes after thyroidectomy. Contemp Surg. 2006;62:410–413. [Google Scholar]
- 41.Ashrafi A S, Sundaresan R S. New York, NY: WebMD; 2006. Dysphagia; pp. 1–9. [Google Scholar]
- 42.Razfar A, Sadr-Hosseini S M, Rosen C A. et al. Prevention and management of dysphonia during anterior cervical spine surgery. Laryngoscope. 2012;122:2179–2183. doi: 10.1002/lary.23284. [DOI] [PubMed] [Google Scholar]
- 43.Winslow C P, Winslow T J, Wax M K. Dysphonia and dysphagia following the anterior approach to the cervical spine. Arch Otolaryngol Head Neck Surg. 2001;127:51–55. doi: 10.1001/archotol.127.1.51. [DOI] [PubMed] [Google Scholar]
- 44.Tervonen H, Niemelä M, Lauri E R. et al. Dysphonia and dysphagia after anterior cervical decompression. J Neurosurg Spine. 2007;7:124–130. doi: 10.3171/SPI-07/08/124. [DOI] [PubMed] [Google Scholar]
- 45.Fountas K N, Kapsalaki E Z, Nikolakakos L G. et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32:2310–2317. doi: 10.1097/BRS.0b013e318154c57e. [DOI] [PubMed] [Google Scholar]
- 46.Edwards C C II, Karpitskaya Y, Cha C. et al. Accurate identification of adverse outcomes after cervical spine surgery. J Bone Joint Surg Am. 2004;86-A:251–256. doi: 10.2106/00004623-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Lee J Y Lim M R Albert T J Dysphagia after anterior cervical spine surgery: pathophysiology, incidence, and prevention. Cervical Spine Research Society Outcome Study, 2005 Available at: http://www.csrs.org/web/outcomes/clinsafetyoutcomespaper.pdf. Accessed February 11, 2013
- 48.Vaidya R, Carp J, Sethi A, Bartol S, Craig J, Les C M. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J. 2007;16:1257–1265. doi: 10.1007/s00586-007-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoo J U, Hiratzka J R. Philadelphia, PA: Lippincott Williams & Wilkins; 2012. Dysphagia and dysphonia; pp. 1326–1331. [Google Scholar]
- 50.Kang S H, Kim D K, Seo K M, Kim K T, Kim Y B. Multi-level spinal fusion and postoperative prevertebral thickness increase the risk of dysphagia after anterior cervical spine surgery. J Clin Neurosci. 2011;18:1369–1373. doi: 10.1016/j.jocn.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 51.Ratnaraj J Todorov A McHugh T Cheng M A Lauryssen C Effects of decreasing endotracheal tube cuff pressures during neck retraction for anterior cervical spine surgery J Neurosurg 200297(2, Suppl):176–179. [DOI] [PubMed] [Google Scholar]
- 52.Siska P A, Ponnappan R K, Hohl J B, Lee J Y, Kang J D, Donaldson W F III. Dysphagia after anterior cervical spine surgery: a prospective study using the swallowing-quality of life questionnaire and analysis of patient comorbidities. Spine (Phila Pa 1976) 2011;36:1387–1391. doi: 10.1097/BRS.0b013e31822340f2. [DOI] [PubMed] [Google Scholar]
- 53.Mummaneni P V, Burkus J K, Haid R W, Traynelis V C, Zdeblick T A. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine. 2007;6:198–209. doi: 10.3171/spi.2007.6.3.198. [DOI] [PubMed] [Google Scholar]
- 54.Stachniak J B, Diebner J D, Brunk E S, Speed S M. Analysis of prevertebral soft-tissue swelling and dysphagia in multilevel anterior cervical discectomy and fusion with recombinant human bone morphogenetic protein-2 in patients at risk for pseudarthrosis. J Neurosurg Spine. 2011;14:244–249. doi: 10.3171/2010.9.SPINE09828. [DOI] [PubMed] [Google Scholar]
- 55.Heese O, Schröder F, Westphal M, Papavero L. Intraoperative measurement of pharynx/esophagus retraction during anterior cervical surgery. Part I: pressure. Eur Spine J. 2006;15:1833–1837. doi: 10.1007/s00586-006-0069-0. [DOI] [PubMed] [Google Scholar]
- 56.Heese O, Fritzsche E, Heiland M, Westphal M, Papavero L. Intraoperative measurement of pharynx/esophagus retraction during anterior cervical surgery. Part II: perfusion. Eur Spine J. 2006;15:1839–1843. doi: 10.1007/s00586-006-0070-7. [DOI] [PubMed] [Google Scholar]
- 57.Chen Z, Wei X, Li F. et al. Tracheal traction exercise reduces the occurrence of postoperative dysphagia after anterior cervical spine surgery. Spine (Phila Pa 1976) 2012;37:1292–1296. doi: 10.1097/BRS.0b013e3182477f26. [DOI] [PubMed] [Google Scholar]
- 58.Apfelbaum R I, Kriskovich M D, Haller J R. On the incidence, cause, and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine (Phila Pa 1976) 2000;25:2906–2912. doi: 10.1097/00007632-200011150-00012. [DOI] [PubMed] [Google Scholar]
- 59.Kriskovich M D, Apfelbaum R I, Haller J R. Vocal fold paralysis after anterior cervical spine surgery: incidence, mechanism, and prevention of injury. Laryngoscope. 2000;110:1467–1473. doi: 10.1097/00005537-200009000-00011. [DOI] [PubMed] [Google Scholar]


